Acute Hepatitis in ED Setting: Etiologies, Evaluation, and Management
- Apr 18th, 2022
- Anna Waller
- categories:
Author: Anna Waller, MD (Assistant Professor of Emergency Medicine, San Antonio, TX) // Reviewed by: Alexander Y. Sheng, MD, MHPE (@TheShenger); Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)
Case 1
25-year-old female nurse presents with low grade fever, malaise, and myalgias over the past 2 weeks. She became concerned today because she noticed that her eyes looked yellow. She has felt nauseated but has not been vomiting. Her vital signs are remarkable for a temperature of 100.4 F and a heart rate of 110. Her exam is unremarkable, and she is nontoxic appearing. On further history, she works in the operating room and had a needle stick 4 weeks ago but did not report it or seek treatment.
Case 2
62-year-old male with history of hypertension, hyperlipidemia presents with abdominal pain and jaundice. He reports that he was diagnosed with bacterial pneumonia 5 weeks ago. His cough has improved, but the abdominal pain began 2 weeks ago, and he reports that his skin has become progressively more yellow since that time.
Introduction
Acute hepatitis is defined as inflammation of hepatic parenchyma or hepatocellular necrosis resulting in increased liver enzymes lasting <6 months (1). Hepatitis occurs when either an infectious or autoimmune process, hepatotoxic substance, or congenital disease result in damage to the liver. In rare cases, acute hepatitis can progress to acute fulminant hepatic failure, characterized by in impairment of liver synthetic function and the presence of hepatic encephalopathy (2). The primary goals of care in the emergency department (ED) setting are to treat symptoms, assess risk for acute fulminant hepatic failure, identify and treat drug-induced liver disease and reversible causes, and rule out acute obstructive cholestatic disease.
Infectious Hepatitis
Hepatitis A is the most common cause of acute viral hepatitis in the United States (US), with 18,849 reported cases in 2019 as identified by the Center for Disease Control and Prevention (CDC) (3). Hepatitis A is transmitted by the fecal-oral route and its incidence in developing countries far exceeds that of the US. It is estimated that 90% of children in countries where hepatitis A is endemic have been infected by the age of 10 (4). The progression to fulminant liver failure is rare, therefore, despite its prevalence, hepatitis A causes only about 0.8% of total mortality from viral hepatitis yearly (5). The introduction of vaccination in the US in 1996 decreased rates of hepatitis A by 95% and the primary group affected are those who have not been vaccinated in childhood (6). Hepatitis E is similar to Hepatitis A in that it is also transmitted via fecal-oral route. It is less common in the US, associated more with sporadic outbreaks of contaminated meat or seafood (3). Worldwide, hepatitis E has a higher mortality than hepatitis A, accounting for 3.3% of fatalities from all viral hepatitis and causes more severe illness in pregnant women (5).
The WHO estimates that 3.5% of the world’s population has chronic hepatitis B (5). Due to improved vaccination in the US with immunization at birth, hepatitis B infections remains low in comparison to developing countries. There were 3,409 new cases in the US in 2017, although it is likely under identified. As of 2016, there were approximately 863,000 people who had chronic hepatitis B infection in the US (7,8). Hepatitis B is transmitted via blood and body fluids through direct contact, parenteral inoculation, sexual contact, and vertical transmission. Vertical transmission remains a large source of infection in the developing world, although in the US, infections are more frequently associated with intravenous drug abuse and high-risk sexual activity in young adults (9,10). Hepatitis D virus may be contracted by patients who already have hepatitis B but is not found in isolation. Hepatitis C is transmitted similarly to Hepatitis B, although with less risk of vertical or sexual transmission compared to hepatitis B (11). Likely under-identified, new reported cases of hepatitis C reached 4,136 in 2019, although the CDC estimates that there were more than 50,000 new cases (7). There are likely 2.5 million people in the US chronically infected with Hepatitis C (7).
Hepatitis A-E remain the most common identified etiology of acute infectious hepatitis, although as shown in Table 1, there are many other infectious agents that cause acute hepatitis. A thorough history of recent travel, sexual activity, exposures will help narrow the differential diagnosis.

Noninfectious Hepatitis
Noninfectious causes of acute hepatitis include hepatotoxic substances and drug reactions, hypotension or shock causing “shock liver,” autoimmune, neoplastic, congenital/heritable causes (Table 2). Drug reactions are the most common cause of acute hepatic failure and a large contributor to acute hepatitis, and therefore, a focus of this article (13).

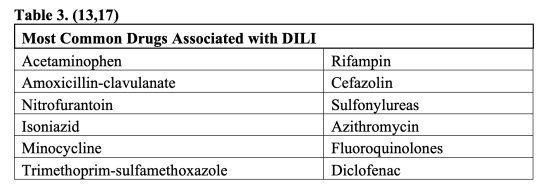
Drug induced liver injury (DILI) is a term used to describe damage to the liver from a form of medication or herbal supplement. The US Food and Drug Administration (FDA) is vigilant to identify and regulate those medications in which DILI may be a relatively common side effect (13). Acetaminophen hepatotoxicity is the predominant cause of acute fulminant hepatic failure in adults, causing 41% of cases in the US, 61% of which were unintentional overdoses (14). DILI caused by acetaminophen usually occurs in a dose dependent and relatively predictable pattern in most individuals through the accumulation of the toxic NAPQI metabolite (15). A thorough history including combination over the counter medications will sometimes reveal that a patient is taking more than the recommended 4000 mg/day. Idiosyncratic drug reactions may be caused by any drug and are characterized by unpredictability, lack of dose-dependent toxicity, variability in timing, and lack of reproducibility in animal models (13). Idiosyncratic reactions may be immune mediated, such as in Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) (13,16). Immune mediated DILI most often occurs 2-8 weeks after the drug was introduced, but many non-immune mediated reactions may occur as far out as one year after drug introduction and even after it has been stopped (13). Although idiosyncratic DILI by nature is unpredictable and varies depend on host factors, it is more likely with longer duration of therapy (13) and there are certain medications and subsets of medications that are more common offenders (Table 3). A prospective study by the American Drug Induced Liver Injury Network found that antibiotics were the most common cause of DILI (45%) (17).
Herbal remedies and dietary supplements have fewer requirements from the FDA and recognized as a large subgroup causing DILI, representing 16% of cases (17,18). Supplements may be removed from the market by the FDA if they are proven to be either harmful or misbranded, but the manufacturer is initially responsible for ensuring product safety. (18). The category of appearance and performance enhancing drugs account for the largest subgroup of drugs containing unlabeled ingredients (19).
Alcohol also causes liver injury in a semi-dose dependent manner and a major cause of acute hepatitis and chronic liver disease (20). There is a threshold above which liver injury is more likely (approximately >2 drinks per day for women and >5 drinks per day in men), but only a subset of heavy alcohol users develops acute alcoholic hepatitis or cirrhosis (20).
History and Exam
Patient presentation varies from asymptomatic elevated transaminases to fulminant hepatic failure, therefore, a high index of suspicion in allcomers to the ED is necessary to identify acute hepatitis. Historical features concerning for hepatitis should be based on common causes of acute hepatitis. Concerning features of history include chronic ingestion of > 4000 mg/day total of acetaminophen, recent long term antibiotic use, use of weight loss supplements, suicide attempts, recent travel to areas endemic of infectious etiologies that commonly cause acute hepatitis, high risk sexual history, intravenous drug abuse, exposure to contaminated groundwater containing carbon tetrachloride, mushroom foraging, etc. Common complaints include general fatigue/malaise, low grade fever, nausea, vomiting, headache, anorexia, abdominal pain. In the later stages of acute hepatitis, jaundice, acholic stools, and dark urine occur, often trigger an ED visit (11).
Exam may reveal hepatomegaly and right upper quadrant abdominal tenderness to palpation. Scleral icterus is present in cholestatic hepatitis and more prevalent as hepatocellular injury progresses (1). As acute hepatitis leads to hepatic failure, patients will present with bruising/easy bleeding, hypotension, confusion/altered mental status.
ED Evaluation
The predominant liver enzymes tested in the ED are aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and bilirubin (total and direct). Liver synthetic function is mainly assessed by its ability to make vitamin K dependent clotting factors (PT/INR) and albumin. Hepatitis is commonly categorized into hepatocellular and cholestatic based on laboratory values as the illness progresses. Hepatocellular hepatitis is associated with a relatively larger increase in AST/ALT compared to ALP/bilirubin (21). Alkaline phosphatase and bilirubin will have a comparatively greater increase in the cholestatic pattern of hepatitis, although this may not be evident on initial evaluation (1, 11, 22). ALT is considered more specific to the liver than AST, which is found in skeletal and cardiac muscle. Elevation of AST>ALT is characteristic of alcohol-related hepatitis, although AST elevation also occurs in thyroid disease, myopathy, exercise. Alcoholic liver disease typically causes an elevation of AST:ALT ratio of >2:1 (23).
Bilirubin is primarily formed by the breakdown of red blood cells and circulates in the unconjugated form while bound to albumin. Conjugation occurs via UDP-glucuronosyltransferase in the liver. Conjugated (direct) bilirubin is elevated when there is hepatocellular damage or cholestasis (24). A predominance of conjugated hyperbilirubinemia occurs when there is either an intrahepatic or extrahepatic obstruction or impairment in bile excretion after it has been metabolized in the liver. This finding warrants advanced imaging in the form of a liver ultrasound, abdominal CT, or magnetic resonance cholangiopancreatography (MRCP) in patients with severe disease or without reliable follow up to specifically exclude malignancy and other causes of cholestasis (Table 4). Choledocholithiasis can lead to ascending cholangitis, if undiagnosed, which is characterized by elevated bilirubin/jaundice, fever, abdominal pain and progresses to altered mental status and hypotension (25). Hepatocellular damage and cholestasis also occur in severe illness/shock, so the differential should extend beyond the liver (26).

In patients with elevated transaminases, hepatic function should be assessed with PT/INR and albumin. Diminished hepatic synthetic function is a marker of poor prognosis in acute viral hepatitis as it signals progression to fulminant hepatic failure. PT measures the liver’s synthetic function of vitamin K dependent coagulation factors and is more specific to the liver than albumin (1). Albumin is decreased by cytokine effects in severe illness, which renders it less reliable (24).
ED laboratory evaluation should also include acetaminophen levels in patients with acute or chronic exposure, other drug levels such as salicylates or antiepileptics, and lactate as needed if concerned for sepsis.
Complications
Acute viral hepatitis caused by hepatitis B or C may lead to chronic hepatitis. The risk of developing chronic hepatitis B infection decreases with age. Ninety percent of infants infected with Hepatitis B will develop chronic infection, while only 2-6% of adults develop chronic infection (7). Approximately 31-50% of patients infected with hepatitis C develop a chronic infection. (30,31). Testing for antibodies and/or antigens to Hepatitis A-E may be done in the hospital or outpatient, and results may not be available while the patient is in the ED. Long term sequelae of chronic hepatitis B and C include hepatocellular carcinoma and cirrhosis. Therefore, prevention, early diagnosis, treatment, and monitoring are important to ensure good long-term health in these patients. Outpatient treatments for chronic hepatitis C may be curative, and patients with risk factors should be screened in the ED or referred for testing. (31,32). Hepatitis B immunoglobulin is not effective in acute or chronic hepatitis B. Hepatitis B immune globulin in combination with Hepatitis B vaccine is recommended within 24 hours of exposure as post exposure prophylaxis in patients who had a known exposure to body fluids containing Hepatitis B surface Ag or for sexual partners of known hepatitis B carriers if it can be given within 14 days of last exposure (33). Of note, household contacts of patients with acute hepatitis B may benefit from the immunoglobulin if not previously vaccinated (33).
The feared complication of acute hepatitis from any cause is the development of acute liver failure. The definition of acute liver failure by the American Association for the Study of Liver Diseases (AASLD) is predicated on new diagnosis (new diagnosis of liver disease and progression less than 26 weeks) and diminished hepatic function (development of coagulopathy [INR >1.5] and hepatic encephalopathy) (34).
The risk of progression to acute liver failure varies based on the underlying etiology, and data are incomplete given the variety of etiologies (Tables 1-3). Specifically in patients with acute hepatitis B, total bilirubin value >5x the upper limit of normal and HBeAg negative status at time of admission have shown to independently increase risk of progression to acute liver failure in a retrospective observational study (30). Other factors may also contribute, as age over 50, elevated viral load and high core antigen levels are suggestive of worse outcome (30).
The FDA applies Hy’s law to extrapolate the risk that a proposed drug will cause drug induced liver injury (DILI) resulting in death or liver transplant based on a small sample size. Even one case of severe DILI in a clinical trial could indicate the risk for large numbers in the general population (35).
Hy’s Law is:
- The drug causes hepatocellular pattern of injury: ALT or AST >3x upper limit of normal.
- Total bilirubin levels are >2xupper limit of normal, without initial findings of cholestasis (elevated serum ALP).
- Other causes of hepatitis have been excluded (viral hepatitis, preexisting or acute liver disease, etc.).
The rate of Hy’s Law cases in a clinical trial can be extrapolated to the general population, usually at 1/10 of the rate noted in the trial. If there are more than 2 cases in a clinical trial, this will delay or eliminate the chance of FDA drug approval (35-37). Although the value of Hy’s law as a predictive tool for the general population has also been debated (38), it is worth noting that this pattern of injury may be associated with poorer outcome. Elevated bilirubin without evidence of obstructive cholestasis indicates that the hepatocellular injury has become so severe that the liver cells are unable to excrete bilirubin, an ominous sign (35). Patients with this type of injury pattern may be higher risk.
Management
Acute management in the ED for hepatitis may include GI decontamination after ingestion, N-acetyl cysteine, stabilization of the patient, empiric treatment of suspected infection, and early referral to liver transplant center, if applicable. All treatments should be considered, although they are only beneficial in a select number of patients. GI decontamination decreases absorption of the offending agent if there was an acute ingestion of a hepatotoxic agent, depending on the agent and the amount ingested, using activated charcoal or whole bowel irrigation.
N-acetyl cysteine (NAC) is used in the treatment of acetaminophen hepatotoxicity to regenerate intracellular glutathione and metabolize NAPQI. In acetaminophen toxicity with high risk of progression to acute liver failure, it’s use has been demonstrated to decrease mortality from 5% to 0.7%. (15,39). NAC should be used empirically and ideally within the first hour of acute acetaminophen ingestion in combination with activated charcoal if the history suggests that there was a large ingestion likely to cause hepatotoxicity (>150 mg/kg) (15). The Rumack-Matthew nomogram guides treatment after a 4-hour acetaminophen level is drawn, with serum acetaminophen levels >150mg/dL warranting treatment. Chronic acetaminophen toxicity should also be treated with NAC if the acetaminophen level is >20mg/dL, the patient is symptomatic, and ALT level is elevated (15).
Despite some literature suggesting improvement in transplant-free survival in acute liver failure not caused by acetaminophen toxicity (40), a 2020 Cochrane review concluded that there is insufficient evidence to warrant empiric treatment with NAC in acute liver failure without acetaminophen toxicity and at worst, administration may delay liver transplant (42, 43). Depending on the expected course of disease and cause of acute hepatitis/liver failure, NAC may still be warranted as in cases of Amanita phalloides hepatotoxicity in combination with IV Penicillin G (2). Several agencies do include liberal use of NAC in their guidelines, so early consultation with a toxicologist or hepatologist may change management in patients with severe disease, although with limited evidence (2, 43, 44). Other treatments including steroids for acute severe alcoholic hepatitis are part of inpatient management and generally deferred until infection has been ruled out (45).
The most common cause of death in acute liver failure stems from multi-organ failure (45). In all patients, standard ED management of severely ill patients applies, with a focus on supportive care and identifying co-existing infections, traumatic injuries, and management of immediate life threats. Early consideration and transfer to a liver transplant center is vital as the 7-day mortality of these patients is high (47). Use of the MELD score for acute severe hepatitis aids in determination of which patients meet criteria for early liver transplant (48).
Case conclusions
Case 1
The patient reports that she had been vaccinated for hepatitis B as a child, although a previous antibody titer was low, and she has not been re-vaccinated. Her AST was noted to be 300 and ALT 425. Alkaline phosphatase is 500. Total bilirubin is 1.7. Her PT/INR and albumin levels are in normal range. Acetaminophen level is negative. Her vital signs improve with supportive care in the ED, and she has a right upper quadrant ultrasound that does not reveal evidence of biliary obstruction. She can eat and drink without discomfort and you arrange close outpatient follow up with her primary doctor. You follow her up later and discovered that she tested positive for hepatitis B, her liver enzymes normalized, and she did not develop chronic infection.
Case 2
The patient’s exam reveals ill appearing man with marked icterus. He has right upper quadrant tenderness without rebound or guarding. His labs reveal AST of 200, ALT of 230, Alkaline phosphatase of 300, total bilirubin of 20, INR of 1.3, albumin is 3.5. Acetaminophen level is negative. His liver ultrasound is negative for evidence of obstructive cholestasis. He is admitted to the hospital. Viral and autoimmune studies are negative. Blood cultures do not grow any microorganisms. He develops worsening liver function as INR increases to 2.0 and he develops hepatic encephalopathy. He is transferred to a liver transplant center. After extensive review of his medications and a liver biopsy, it is determined that his acute hepatitis and subsequent liver failure was due to idiosyncratic reaction to amoxicillin-clavulanate. His liver function improves over the next few months, and he does not require a liver transplant.
Clinical Pearls
- Take a careful history to include risk factors for acute viral hepatitis, alcoholic hepatitis, prescription medications, supplements, and combination over-the-counter medicines that include acetaminophen as chronic toxicity is often unintentional.
- In patients with acute hepatitis, assess for diminished synthetic activity of liver function and refer to a liver transplant center if the disease has progressed to acute liver failure.
- Consider and exclude acute obstructive cholestasis as the cause for elevated transaminases and bilirubin.
- Consider the risk for progression to acute liver failure in all patients with acute hepatitis. Although this is a rare complication, it is more likely in patients with significant elevations in bilirubin without evidence of obstruction.
References
- Schaefer TJ, John S. Acute Hepatitis. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551570/
- Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55(3):965-967. doi:10.1002/hep.25551
- org [Internet]. Hepatitis A. Centers for Disease Control and Prevention; 2020, Jun 20 [cited 2022 Jan 25]. Available from: https://www.cdc.gov/hepatitis/hav/index.htm
- Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28(41):6653-6657. doi:10.1016/j.vaccine.2010.08.037
- World Health Organization. Global hepatitis report 2017. World Health Organization, 2017.
- Foster MA, Haber P, and Nelson NP. Hepatitis A. Epidemiology and Prevention of Vaccine-Preventable Diseases 14thEdition [Internet]. 2021 [cited 2022 Apr 12]; from: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/hepa.pdf
- Viral Hepatitis Surveillance, United States 2019 [internet]. Centers for Disease Control and Prevention; 2019, Nov 14 [cited 2022 Jan 25]. Available from: https://www.cdc.gov/hepatitis/statistics/2019surveillance/index.htm
- LeFevre ML. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: US Preventive Services Task Force recommendation statement. Annals Internal Med. 2014;161(1):58–66.
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546-1555. doi:10.1016/S0140-6736(15)61412-X
- Jefferies M, Rauff B, Rashid H, Lam T, Rafiq S. Update on global epidemiology of viral hepatitis and preventive strategies. World J Clin Cases. 2018;6(13):589-599. doi:10.12998/wjcc.v6.i13.589
- Kochar R, Sheikh AM, Fallon MB. Acute and Chronic Hepatitis. In: Andreoli TE, Benjamin IJ, Griggs RC, Wing EJ, editors. Andreoli and Carpenter’s Cecil Essentials of Medicine 8th Edition. Saunders Elsevier; 2010. p. 446-475.
- Kwong S, Meyerson C, Zheng W, et al. Acute hepatitis and acute liver failure: Pathologic diagnosis and differential diagnosis. Semin Diagn Pathol. 2019;36(6):404-414. doi:10.1053/j.semdp.2019.07.005
- Katarey D, Verma S. Drug-induced liver injury. Clin Med (Lond). 2016;16(Suppl 6):s104-s109. doi:10.7861/clinmedicine.16-6-s104
- Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure [published correction appears in Am J Gastroenterol. 2008 Jan;103(1):255]. Am J Gastroenterol. 2007;102(11):2459-2463. doi:10.1111/j.1572-0241.2007.01388.x
- Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N. Acetaminophen-Induced Hepatotoxicity: A Comprehensive Update. J Clin Transl Hepatol. 2016;4(2):131-142. doi:10.14218/JCTH.2015.00052
- Choudhary S, McLeod M, Torchia D, Romanelli P. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome. J Clin Aesthet Dermatol. 2013;6(6):31-37.
- Chalasani N, Bonkovsky HL, Fontana R, et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148(7):1340-52.e7. doi:10.1053/j.gastro.2015.03.006
- gov [internet]. Food and Drug Administration. 2019 Aug 16. [cited 2022 Jan 25] Available from: https://www.fda.gov/food/dietary-supplements
- Navarro V, Avula B, Khan I, et al. The Contents of Herbal and Dietary Supplements Implicated in Liver Injury in the United States Are Frequently Mislabeled. Hepatol Commun. 2019;3(6):792-794. Published 2019 Apr 3. doi:10.1002/hep4.1346
- Gholam PM. Prognosis and Prognostic Scoring Models for Alcoholic Liver Disease and Acute Alcoholic Hepatitis. Clin Liver Dis. 2016;20(3):491-497. doi:10.1016/j.cld.2016.02.007
- Lala V, Goyal A, Bansal P, et al. Liver Function Tests. [Updated 2021 Aug 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482489/
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Acute Hepatitis. [Updated 2019 May 4.
- Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94(4):1018-1022. doi:10.1111/j.1572-0241.1999.01006.x
- Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017 Jan;112(1):18-35. [PubMed: 27995906]
- Hanau LH, Steigbigel NH. Acute (ascending) cholangitis. Infect Dis Clin North Am. 2000;14(3):521-546. doi:10.1016/s0891-5520(05)70119-7
- Wang D, Yin Y, Yao Y. Advances in sepsis-associated liver dysfunction. Burns Trauma. 2014;2(3):97-105. Published 2014 Jul 28. doi:10.4103/2321-3868.132689
- Shah R, John S. Cholestatic Jaundice. [Updated 2021 Jul 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482279/
- Pollock, G., and Minuk, G. Y.(2017) Diagnostic considerations for cholestatic liver disease. Journal of Gastroenterology and Hepatology, 32: 1303– 1309. doi: 1111/jgh.13738
- Assy N, Jacob G, Spira G, Edoute Y. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5(3):252-262. doi:10.3748/wjg.v5.i3.252
- Xiong QF, Xiong T, Huang P, Zhong YD, Wang HL, Yang YF. Early predictors of acute hepatitis B progression to liver failure. PLoS One. 2018;13(7):e0201049. Published 2018 Jul 26. doi:10.1371/journal.pone.0201049
- Seo S, Silverberg MJ, Hurley LB, et al. Prevalence of Spontaneous Clearance of Hepatitis C Virus Infection Doubled From 1998 to 2017. Clin Gastroenterol Hepatol. 2020;18(2):511-513. doi:10.1016/j.cgh.2019.04.035
- Mir F, Kahveci AS, Ibdah JA, Tahan V. Sofosbuvir/velpatasvir regimen promises an effective pan-genotypic hepatitis C virus cure. Drug Des Devel Ther. 2017;11:497-502. Published 2017 Feb 23. doi:10.2147/DDDT.S130945
- Habib, Shahid and Obaid S. Shaikh. Hepatitis B immune globulin. Drugs Today (Barc). 2007 Jun;43(6):379-94. doi: 10.1358/dot.2007.43.6.1050792.
- Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55(3):965–967.
- Guidance for Industry Drug-Induced Liver Injury: Premarketing Clinical Evaluation. Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research. 2009 Jul. Docket Number: FDA-2008-D-0128. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-induced-liver-injury-premarketing-clinical-evaluation
- Andrade RJ, Lucena MI, Fernández MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period [published correction appears in Gastroenterology. 2005 Nov;129(5):1808]. Gastroenterology. 2005;129(2):512-521. doi:10.1016/j.gastro.2005.05.006
- Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42(2):481-489. doi:10.1002/hep.20800
- Almomen S, M, Almaghrabi M, A, Alhabardi S, M, Alrwisan A, A: Exploring Indicators of Hepatotoxicity-Related Post-Marketing Regulatory Actions: A Retrospective Analysis of Drug Approval Data. Saudi J Health Syst Res 2022. doi: 10.1159/000521296
- Chun LJ, Tong MJ, Busuttil RW, Hiatt JR. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43(4):342-349. doi:10.1097/MCG.0b013e31818a3854
- Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure [published correction appears in Gastroenterology. 2013 Sep;145(3):695. Dosage error in article text]. Gastroenterology. 2009;137(3):856-864.e1. doi:10.1053/j.gastro.2009.06.006
- Siu JT, Nguyen T, Turgeon RD. N-acetylcysteine for non-paracetamol (acetaminophen)-related acute liver failure. Cochrane Database Syst Rev. 2020 Dec 9;12(12):CD012123. doi: 10.1002/14651858.CD012123.pub2. PMID: 33294991; PMCID: PMC8095024.
- Aljohani W, Chan BPH, Yaghoobi M. Role of N-Acetylcysteine in the Treatment of Acute Nonacetaminophen, Nonalcoholic and Nonviral Hepatitis: A Meta-analysis. J Can Assoc Gastroenterol. 2020;4(3):125-130. Published 2020 Jul 15. doi:10.1093/jcag/gwaa017
- Benić MS, Nežić L, Vujić-Aleksić V, Mititelu-Tartau L. Novel Therapies for the Treatment of Drug-Induced Liver Injury: A Systematic Review. Front Pharmacol. 2022;12:785790. Published 2022 Feb 2. doi:10.3389/fphar.2021.785790
- European Association for the Study of the Liver Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70(6):1222-1261. doi:10.1016/j.jhep.2019.02.014
- Dong V, Nanchal R, Karvellas CJ. Pathophysiology of Acute Liver Failure. Nutr Clin Pract. 2020;35(1):24-29. doi:10.1002/ncp.10459
- Hosseini N, Shor J, Szabo G. Alcoholic Hepatitis: A Review. Alcohol Alcohol. 2019;54(4):408-416. doi:10.1093/alcalc/agz036
- Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394(10201):869-881. doi:10.1016/S0140-6736(19)31894-X
- Montrief T, Koyfman A, Long B. Acute liver failure: A review for emergency physicians. Am J Emerg Med. 2019;37(2):329-337. doi:10.1016/j.ajem.2018.10.032
