ED Management of Heart Failure: Pearls and Pitfalls
- Nov 22nd, 2016
- Kristine Jeffers
- categories:
Authors: Kristine Jeffers, MD (EM Resident at SAUSHEC) and Brit Long, MD (@long_brit, EM Attending Physician at SAUSHEC // Edited by: Alex Koyfman, MD (@EMHighAK, EM Attending Physician, UTSW / Parkland Memorial Hospital)
Case:
A 68-year-old male presented to the emergency department with chief complaint of dyspnea on exertion that had been worsening over the last week. Initial vitals were BP 120/100, HR 142, RR 22, T98.0, and Sat 96% on RA. An ECG obtained at triage is shown below.
On further questioning he reported general malaise over the last month and progressive dyspnea with exertion over the last week. He was no longer able to walk more than about 15 feet and used to care for his farm himself. He also reported significant swelling in his lower extremities that had been worsening over the last 4 days. He denied any medical problems or taking any medicines but had not been to a physician in over 20 years.
On physical exam, he was tachycardic with an irregularly irregular rhythm and no murmurs rubs or gallops, his lungs had crackles bilaterally, and he was mildly tachypneic. His abdomen was soft and nontender. His lower extremities had 2+ pitting edema to above the knees, and his pules were 2+ distally.
A portable CXR showed:
The patient’s symptoms were thought to be secondary to atrial fibrillation with rapid ventricular response. Due to his apparent fluid overload and unknown ejection fraction (EF), the patient was given 5mg of Metoprolol IV. This brought his heart rate down to 100-110. He was loaded with 100mg PO metoprolol at that time.
About 45 minutes after his heart rate had slowed, the patient appeared to be becoming more uncomfortable. He developed mild respiratory distress and became hypotensive. He was placed on BIPAP, and his blood pressure dropped to 55/24. At this time his extremities had become cool, and he was altered and agitated. A quick bedside ECHO showed diffuse hypokinesis. A central line and arterial line were placed, while push dose epinephrine was given to maintain perfusion. Once the central line was in place, he was started on a dobutamine at 10mcg/kg/min and norepinephrine. At this time cardiology recommended emergent cardioversion, as the patient was persistently in atrial fibrillation (rate 110-120) with hypotension. He was given a small dose (40mg) of propofol and cardioverted with 200J with successful return to NSR. At that time he was transferred to the ICU for further care.
Background
Acute heart failure (AHF) is a disease characterized by inadequate blood flow to meet metabolic demands. It often includes rapid onset of symptoms and signs secondary to abnormal cardiac function.1 AHF is a disease associated with high morbidity and mortality. It accounts for over 650,000 emergency department (ED) visits annually, with over 80% leading to admission.2 Those hospitalized are at high risk, with over one third of patients dying or requiring repeat admission within 90 days. In addition to the high burden of disease, AHF poses significant costs to the health system with over $39 billion per year.3
Pathophysiology
CHF ultimately develops from cardiac dysfunction, which leads to decreased cardiac output from myocardial stress or injury. Anything that threatens cardiac output triggers a cascade activating the renin-angiotensin-aldosterone and sympathetic nervous systems. This leads to increased levels of norepinephrine, vasopressin, endothelin, and TNF-alpha, which result in sodium and water retention. This further increases systemic vascular resistance, cardiac workload, wall tension, and O2 demand. Over the long term this leads to cardiac remodeling, contributing to the cycle of congestive heart failure.3
Diagnosis
The diagnosis of ACHF is based on symptoms and clinical findings supported by appropriate investigations. Possible tests include ECG, CXR, electrolytes, liver function, biomarkers, and echocardiogram. Early diagnosis and initiation of treatment improves outcome.2 The following are suggestive of heart failure:
-History: Dyspnea, orthopnea, fatigue, weakness, leg swelling, and abdominal swelling.6
-Physical Exam: Respiratory distress, rales, S3, JVD, hepatojugular reflux, hepatomegaly, ascites, edema, diaphoresis, tachycardia, hypoxia.4
-ECG: Often abnormal in heart failure. Identify the rhythm and assess for ACS, signs of strain, and dysrhythmia.
-CXR: Look for pulmonary congestion, effusions, and cardiomegaly. Also assess for focal consolidation or pneumothorax, which are other causes of dyspnea. Keep in mind that up to 20% of patients have no signs of congestion on CXR.2
Below is a summary of diagnostic accuracy of findings on chest radiograph and ECG for AHF in ED patients presenting with dyspnea.7
-Laboratory evaluations: VBG (pH, lactate), CBC, UA, electrolytes, LFTs, BNP, and troponin.
-BNP: This biomarker is a peptide released from cardiac ventricles in response to increased wall stretch and volume overload. During flash pulmonary edema levels may be normal, as rapid overload is not reflected by BNP elevation. It is also affected by renal failure, older age, female gender, and sepsis (all which increase BNP). Obesity can lower BNP levels. BNP demonstrates a strong predictive value of 90-day outcomes and correlation with in-hospital mortality as BNP worsens.2,6-8,20
-2007 AHFS Guidelines from ACEP recommend at level B obtaining a BNP or pro-BNP, which can improve diagnostic accuracy.22 It is also recommended as a class I recommendation by ACCF/AHA and JFSA guidelines where there is an uncertainty of the diagnosis.
ACEP recommends the following: BNP <100 pg/dL or NT-proBNP <300 pg/dL suggests a diagnosis other than acute heart failure syndrome, while a BNP >500 pg/dL or NT-proBNP >1,000 pg/dL suggests acute heart failure syndrome.22
– Ultrasound: 3+ B-lines in one viewing field (from water-thickened interlobular septa at pleural line) can be used to diagnose pulmonary edema with varying sensitivity and specificity. Classically unilateral symptoms are more suggestive of pneumonia, while bilateral symptoms are thought to be cardiogenic.2 A review of recent literature on this subject with anywhere from novice users to US trained emergency physicians demonstrates 87-94% sensitivity and 49-92% specificity for the diagnosis of acute heart failure.8-14,21 Investigators also found improved accuracy when ultrasound was added to the history and physical exam, compared to gestalt alone.8-14,21
-Echo: assess EF, wall motion, IVC, and lung zones (b-lines).1
Classification:
Patients presenting in heart failure can be placed into four basic categories based on their level of congestion and their level of perfusion. As seen below in the diagram, patients can present from dry and warm (normal) to cold and wet (cardiogenic shock). Patients presenting in the warm and wet category are the most common, but patients can present in shock without being fluid overloaded. Therapy must be tailored to where a patient falls on the spectrum below.4,5
Warm and Wet: AHF and Acute Pulmonary Edema
Acute Congestive Heart Failure Exacerbation
This is one of the most common types of CHF encountered in the emergency department. These patients present with a more indolent course, endorsing weight gain, edema, and dyspnea that worsens over days to weeks.1, 5 These patients may have reduced EF and history of CAD.2 These patients should be evaluated as discussed above, but the mainstay of acute treatment for them is diuresis.
Per the ACEP policy on AFH released in 2007 in which initiation of diuretics in the emergency department is addressed, there is a Class C recommendation that diuretics should be administered judiciously given the potential association of diuretics and worsening renal function at index hospitalization, which affects long-term mortality.22
Acute Pulmonary Edema
Patients may present in acute pulmonary edema, hypertension, and respiratory distress when their symptoms suddenly worsen. An increased 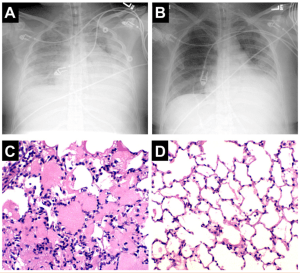 afterload often from elevated vascular pressures leads to hydrostatic lung edema as is seen in the images with initial presentation on the left and resolving fluid on the right.15 These patient present with respiratory distress, tachypnea, rales, crackles, orthopnea, and hypoxia. They are usually hypertensive.
afterload often from elevated vascular pressures leads to hydrostatic lung edema as is seen in the images with initial presentation on the left and resolving fluid on the right.15 These patient present with respiratory distress, tachypnea, rales, crackles, orthopnea, and hypoxia. They are usually hypertensive.
Treatment for pulmonary edema focuses on preload and afterload reduction, followed by diuresis. Vasodilators are the mainstay for treating and relieving symptoms.19 Contraindications for vasodilators include flow limiting preload dependent states like RV infarct, aortic stenosis, or HOCM.
The 2007 emergency medicine practice guidelines also address diuretics, vasodilators, and noninvasive positive pressure ventilation.26
- Vasodilators: There is a level B recommendation to treat patients presenting in AHF with associated dyspnea with IV nitrates. They have a level C recommendation that recommends nitroglycerin over nesiritide, as there is no clear evidence that nesiritide is superior. They state nesiritide should not be considered first line therapy. They also state that ACE inhibitors may be useful in the initial management of acute heart failure, but patients must be monitored for hypotension from the first dose.26
- Nitroglycerin can be given sublingual or IV. Sublingual is useful while setting up a drip and delivers 400mcg over 5 minutes (close to 60-80mcg per minute). A nitroglycerin IV bolus can be given for hypertensive patients, ranging from 100 micrograms to 1mg, often dependent on the provider and institution. A drip should then be started and rapidly increased to reduce preload and afterload. This medication is essential for this category.
- Nitroprusside is only given in a drip form. Nitroprusside should be started at 0.3mcg/kg/min IV and can be titrated up to 5 mcg/kg/minute. However, nitroglycerin properly dosed should improve blood pressure.
- Nicardipine has been shown to increase cardiac output and decrease pulmonary artery pressures, leading to increased cardiac index. Doses used were 1-3 mcg/kg/min IV. Nicardipine may be effective as a potent vasodilator in the initial management of acute heart failure but should not be continued long term due to its effect on the renin-aldosterone-angiotensin system. Further study is warranted for nicardipine use in HTN heart failure.27
- Diuretics: There is a level B recommendation to treat patients with moderate-to-severe pulmonary edema resulting from AHF with furosemide in combination with nitrate therapy. They also have a level C recommendation that monotherapy with diuretics in this setting is unlikely to prevent the need for endotracheal intubation, as opposed to monotherapy with nitrates.22 If the patient is hypertensive with pulmonary edema, use nitroglycerin first to improve fluid distribution and pulmonary edema, followed by diuretics after.
The initial starting dose for furosemide/Lasix varies in the literature, but most recommend initial dosing equal to the home dose in IV form, or 20mg IV for those who are furosemide naive.
– Noninvasive positive pressure ventilation: The level B recommendation is to use 5-10mmHg of CPAP for dyspneic patients with AHF without hypotension or the need for emergent intubation to improve heart rate, respiratory rate, blood pressure, and reduce the need for intubation. This treatment can reduce in-hospital mortality. The level C recommendation states BiPAP could also be considered as an alternative to CPAP.1-3,7,19
Cold and Wet: Cardiogenic Shock
Cardiogenic shock is an acute state of decreased cardiac output, resulting in inadequate tissue perfusion despite adequate or excessive circulating volume. This represents only a small subset of patients presenting in AHF (6-8%) but has a 50% mortality rate (half of those occur in the first 48 hours from diagnosis).1,5,16
Risk factors include massive MI (#1 cause), acute mitral regurgitation, VSD, free wall rupture, RV infarct, sepsis, myocarditis, cardiomyopathy, cardiac contusion, aortic stenosis, HOCM, mitral stenosis, myxoma, tamponade, and chordae rupture.16
The pathophysiology is poorly understood and variable based on the cause of shock. In post myocardial infarction it is thought that a release of inflammatory mediators like cytokines and nitric oxide synthase leads to high levels of nitric oxide. This in turn inhibits contractility, produces a decreased response to catecholamines, and induces vasodilation.
Physical examination will reveal signs of hypoperfusion. The patient may exhibit altered mental status, decreased urine output, systolic blood pressure <90 mmHg, a narrowed pulse pressure of <20, tachycardia, tachypnea, rales, JVD, hepatojugular reflex, peripheral edema, diaphoresis, or a new heart murmur.
The diagnosis is largely clinical based on signs of poor cardiac output and tissue hypoperfusion in addition to signs of fluid overload.
The treatment of cardiogenic shock in the emergency department focuses on temporizing measures until more definitive management correcting the inciting event can occur (surgery, cath lab/PCI…).
-Airway: There is no need to withhold O2 from these patients. Supplemental oxygen, NIPPV, or intubation may be required to maintain adequate oxygenation pending respiratory failure. Preoxygenation is essential for these patients, so start supplemental oxygen right away. Have low threshold to start NIPPV, which can rapidly improve these patients. One thing to consider first is that positive pressure from intubation, which further decreases preload and cardiac output, can worsen hypotension. Be prepared with fluids, pressors, and inotropes if intubation is needed.
-Stabilization: Go back to the basics… Establish your safety net of IV access, supplemental oxygen, and monitors. Consider invasive monitoring with central line and arterial line. Patients may even benefit specific therapies including intra-aortic balloon pump or early PCI, so get your consultants on board early!1,16
-Hypotension: Treatment should be guided by clinical findings. Your decision for fluids vs inotropes (dobutamine) vs pressors (norepinephrine) should be guided by clinical findings.1,16 In those with hypotension, norepinephrine should be started. Once blood pressure stabilizes, then dobutamine can be added.
Cold and Dry
Patients presenting in a low cardiac output state without fluid overload are rare and surprisingly stable clinically. They do not often present with acute symptoms. Adjustments to oral medications are not helpful unless there are problems with filling pressure or excessive vasodilation. Use caution in starting inotropes, as these patients may become dependent or develop tachyphylaxis.5 Beta-blockers if tolerated are associated with later clinical improvement, but there is little evidence and few trials focusing on this group.5 These patients will require cardiology consultation and likely inpatient admission.
Risks of poor outcome
Risks of poor outcome in patients with acute decompensated heart failure include a history of prior hospitalization, markedly elevated BNP (>1000 pg/ml), sodium <136mmol/L, BUN >43mg/dl, SPB <115, or positive troponin. Admission to a critical care unit may be needed for these patients.
In the setting of AHF, SBP demonstrates a differential prognostic association with mortality according to LVEF status. When EF is <40%, SBP is inversely and linearly associated with mortality, whereas patients with a LVEF 50% demonstrate a J-curve pattern, with the lowest risk being in the SBP category 160–179 mmHg and a progressive increased risk of mortality above and below these values.17
Disposition
Over 80% of patients with acute heart failure will be admitted to the hospital. There are few recommendations and no national guidelines outlining who may be appropriate for discharge. Determining who is high versus low risk is difficult, and many of these patients are at high risk of mortality.20,23,25
The Ottawa heart failure risk scale is one such tool that has been proposed. It is based on a retrospective analysis of patients seen in the ED with heart failure. The goal of the score is to identify patients at high risk of serious adverse outcomes (SAE). The score uses 10 pieces of data, worth up to 15 points. Components of the history,  physical, and lab results are used. Patients who score 0 are considered low risk of adverse outcome, with 2.8% risk of SAE. Depending on the cutoff used for admission, whether 0, 1, or 2 points, admission rates can be reduced. By using a score of 2 for admission, you maintain an 80% sensitivity for SAE and would admit approximately 50% of patients, as opposed to the current average in the United States (close to 80% of patients).
physical, and lab results are used. Patients who score 0 are considered low risk of adverse outcome, with 2.8% risk of SAE. Depending on the cutoff used for admission, whether 0, 1, or 2 points, admission rates can be reduced. By using a score of 2 for admission, you maintain an 80% sensitivity for SAE and would admit approximately 50% of patients, as opposed to the current average in the United States (close to 80% of patients).
Several papers have been published investigating decision tools to decide who is high and who is low risk and appropriate for discharge. The problem with many of these tools is they are retrospectively based, and very few have been externally validated.7,8 More work needs to be done to evaluate low risk heart failure patients in the ED. One possible way to utilize a scoring tool would be to use them in combination with an observation unit.
Some have recommended a short course in the ED or even an ED-based observation unit where many of the chronic (but worsened and overloaded) patients could be managed without full admission.18,24 These observation units have the possibility of reducing cost and admission rates, and many ED physicians are knowledgeable in the initial management of acute heart failure syndrome. Many of the factors of inpatient versus outpatient management are based on the individual and the institution. It will require a collaboration of both inpatient and outpatient care to provide comprehensive treatment for these patients.25 Early follow up for these patients has been shown to have a positive impact on 30-day readmission rates, reinforces medication compliance, and allows for patient education.6,7
Pharmacotherapy
A variety of agents have been mentioned in this post that are available for management of these patients. A summary of the dose and mechanism of action of these medications is shown below, as well as their site of action.
| Diuretics | Medication | Dose | Mechanism | Other |
| Lasix/furosemide | IV >/= to home dose of oral | Inhibits Na/Cl reabsorption in the loop of Henle | Ototoxicity, hyperuricemia, hyperglycemia | |
| Bumetanide | 0.5-2 mg/dose (max10mg/day) | 40x more potent than lasix | ||
| Torsemide | 10-20mg Q2H | |||
| Vasodilators | Nitroglycerin | 20-200mcg/minute | Relieves pulmonary congestion. Reduces preload and afterload without impairing tissue Perfusion | Headache, methemoglobinemia with high doses |
| Nitroprusside | 0.3 mcg/kg/min and up | causes cyanide toxicity, hypotension | ||
| Inotropes | Dopamine | <2 mcg/kg/min | Dopaminergic receptors. | Used for hypoperfusion unresponsive to other therapies. Increases contractility |
| >2 mcg/kg/min | Beta receptor agonist | |||
| >5 mcg/kg/min | Alpha receptor agonist | |||
| Dobutamine | 2-20 mcg/kg/min up to 40 mcg/kg/min | Beta receptor agonist | Initial choice in cardiogenic shock. If on beta blocker may need high doses to restore inotropic effect. | |
| PDE Inhibitors | Milrinone | 25 mcg/kg bolus then 0.375-0.75 mcg/kg/min | Type III phosphodiesterase inhibitor | alternative agent in cardiogenic shock refractory to other agents |
| Vasopressors | Epinephrine | 1mcg/min up to 35 mcg/min | Catecholamine, alpha and beta receptors | Pressor of choice in anaphylactic shock. 2nd choice after norepi |
| Norepinephrine | 2-4 mcg/min up to 100mcg/min | Alpha and beta adrenergic | Vasopressor of choice in septic, cardiogenic and hypovolemic shock | |
| Phenylephrine | 20-80 mcg/min up to 360mcg/min | Alpha adrenergic | Initial pressor when limited by tachycardia, Decreases stroke volume and cardiac output | |
| Cardiac Glycosides | Digitalis | 0.125-0.25mg | Inhibits Na/K ATPase-> increases intracellular Ca | Indicated if LV dysfunction with continued NYHA-FC II, III, or IV symptoms |
Summary
–Congestion will be absent from chest x-rays about 20% of the time… don’t be fooled. Bedside US can really benefit, and BNP may be used to help confirm the diagnosis in these patients.
-Ultrasound is a great new addition to confirming the diagnosis of AHF.
-The vast majority of patients presenting with AHF fall into the warm and wet category. Mainstay of treatment for these patients is diuresis. Start with their home dose of furosemide IV.
-Patients presenting in acute pulmonary edema are going to respond best to vasodilators (nitrates), as diuretics will have little effect acutely.
–BiPAP or CPAP should be used in the dyspneic patient.
-Cardiogenic shock is rare but life threatening: Remember your ABCs.
-Positive pressure from intubation further decreases preload and cardiac output which can worsen hypotension. Be prepared to counteract this (push dose epi… norepinephrine… dobutamine).
-Dobutamine is first choice for low cardiac output.
-Norepinephrine is the first choice vasopressor.
References/Further Reading
- Nieminen M, Bohm M, Cowie M, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure. Eur Heart J. 2005;26:384-416.
- Peacock WF, Cannon CM, Singer AJ, Hiestand BC. Considerations for initial therapy in the treatment of acute heart failure. Crit Care. 2015;19(399). doi:10.1186/s13054-015-1114-3.
- Peacock WF. Congestive Heart Failure and Acute Pulmonary Edema. In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, Seventh Edition. ; 2011:405-415.
- Thomas SS, Nohria A. Hemodynamic classifications of Acute Heart Failure and their Clinical Application. Circ J. 2012;76:278-286.
- Nohria A, Lewis E, Stevenson LW. Medical Management of Advanced Heart Failure. J Am Med Assoc. 2002;287(5):628-640.
- Fermann GJ, Collins SP. Initial Management of Patients with Acute Heart Failure. Heart Fail Clin. 2013;9(3):291-306. doi:10.1016/j.hfc.2013.04.004.
- Collins SP, Storrow AB, Levy PD, et al. Early Management of Patients With Acute Heart Failure: State of the Art and Future Directions: A Consensus Document from the SAEM/HFSA Acute Heart Failure Working Group. Acad Emerg Med. 2015;22(1):94-112. doi:10.1111/acem.12538.
- Otterness K, Milne WK, Carpenter CR. Hot off the Press: B-lines and Focused Lung Ultrasound to Diagnose Acute Heart Failure in Dyspneic Patients. Acad Emerg Med. 2015;22(9):1122-1124. doi:10.1111/acem.12751.
- Liteplo AS, Marill KA, Villen T, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): Sonographic B-lines and N-terminal Pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med. 2009;16(3):201-210. doi:10.1111/j.1553-2712.2008.00347.x.
- Vitturi N, Soattin M, Allemand E, Simoni F, Realdi G. Thoracic ultrasonography: A new method for the work-up of patients with dyspnea. J Ultrasound. 2011;14(3):147-151. doi:10.1016/j.jus.2011.06.009.
- Pirozzi C, Numis FG, Pagano A, Melillo P, Copetti R, Schiraldi F. Immediate versus delayed integrated point-of-care-ultrasonography to manage acute dyspnea in the emergency department. Crit Ultrasound J. 2014;6(1):5. doi:10.1186/2036-7902-6-5.
- Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-Care Multi-Organ Ultrasound Improves Diagnostic Accuracy in Adults Presenting to the Emergency Department with Acute Dyspnea. West J Emerg Med. 2016;17(1):46-53. doi:10.5811/westjem.2015.11.28525.
- Gallard E, Redonnet JP, Bourcier JE, et al. Diagnostic performance of cardiopulmonary ultrasound performed by the emergency physician in the management of acute dyspnea. Am J Emerg Med. 2015;33(3):352-358. doi:10.1016/j.ajem.2014.12.003.
- Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014;21(8):843-852. doi:10.1111/acem.12435.
- Matthay MA. Resolution of pulmonary edema thirty years of progress. Am J Respir Crit Care Med. 2014;189(11):1301-1308. doi:10.1164/rccm.201403-0535OE.
- Weber JE, W. Frank Peacock. Cardiogenic Shock. In: Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, Seventh Edition. ; 2011:385-389.
- Nunez J, Nunez E, Fonarow GC, et al. Differential prognostic effect of systolic blood pressure on mortality according to left-ventricular function in patients with acute heart failure. Eur J Heart Fail. 2010;12:38-44. doi:10.1093/eurjhf/hfp176.
- Weintraub NL, Collins SP, Pang PS, et al. Acute Heart Failure Syndromes: Emergency Department Presentation, Treatment, and Disposition: Current Approaches and Future Aims: A Scientific Statement from the American Heart Association. 2010. doi:10.1161/CIR.0b013e3181f9a223.
- Fenwick R. Management of acute heart failure in the emergency department. Emerg Nurse. 2015;23(8):26-35.
- Cohen-Solal A, Laribi S, Ishihara S, et al. Prognostic markers of acute decompensated heart failure: The emerging roles of cardiac biomarkers and prognostic scores. Arch Cardiovasc Dis. 2015;108:64-74. doi:10.1016/j.acvd.2014.10.002.
- Rempell JS, Noble VE. Using lung ultrasound to differentiate patients in acute dyspnea in the prehospital emergency setting. Crit Care. 2011;15(3):161. doi:10.1186/cc10226.
- Silvers SM, Howell JM, Kosowsky JM, Rokos IC, Jagoda AS. Clinical Policy: Critical Issues in the Evaluation and Management of Adult Patients Presenting to the Emergency Department with Acute Heart Failure Syndromes. Ann Emerg Med. 2007;49(5):627-669. doi:10.1016/j.annemergmed.2006.10.024.
- Collins S, Hiestand B. Confounded by hospitalization: risk stratification and admission decisions in emergency department patients with acute heart failure. Acad Emerg Med. 2013;20(1):106-107. doi:10.1111/acem.12045.
- Schrock JW, Emerman CL. Observation Unit Management of Acute Decompensated Heart Failure. Heart Fail Clin. 2009;5(1):85-100. doi:10.1016/j.hfc.2008.08.015.
- Collins SP, Storrow AB. Acute Heart Failure Risk Stratification: Can We Define Low Risk? Heart Fail Clin. 2009;5(1):75-83. doi:10.1016/j.hfc.2008.08.010.
- Stiell IG, Clement CM, Brison RJ, et al. A risk scoring system to identify emergency department patients with heart failure at high risk for serious adverse events. Acad Emerg Med. 2013;20(1):17-26. doi:10.1111/acem.12056.
- McFarland, Scott and Silvers S. Clinical Policy: Management of Acute Heart Failure Syndromes. ACEP News. https://www.acep.org/Clinical—Practice-Management/Clinical-Policy–Management-of-Acute-Heart-Failure-Syndromes/. Published 2007. Accessed February 11, 2016.
- Curran MP, Robinson DM, Keating GM. Intravenous Nicardipine Its Use in the Short-Term Treatment of Hypertension and Various Other Indications. Drugs. 2006;66(13):1755-1782.






Thanks for a good review. It appears that this patient´s life was saved, but as discussed in the post, maybe not using the most ideal approach to CHF pulmonary edema.
One of the first rules to consider with tachycardia is to determine if the patient is ill from the tachycardia, or tachycardic because he/she is ill. Usually this is determined by the cut-off 150 bmp. Someone who is below 150, like this patient was, is unlikely to be ill primarily from the tachycardia.
The symptoms, signs and CXR reported in this case all point to CHF pulmonary edema with fluid overload as the patients primary condition. A reasonable approach might have been to give diuretics, nitrate and CPAP and observe if the tachycardia improved.
Another thing to discuss here is that the rhythm appears to have been determined from the 12 lead EKG only. The 12 lead is only a snapshot, ideal to determine axis, intervals and morphology. To determine rhythm in complex cases you need to keep an eye on the monitor for longer periods, perhaps try vagal manouvers and often you can then see a brief period of 3:1 flutter or other diagnostic clues. When reviewing the EKG, it certainly is most consistent with AF. In V1 there are however suspiciously regular waves that could be consistent with P-waves. I would suggest to reivew the monitor here.
Apart from tho focus of the post, how to manage acute CHF, a side topic of this is how we mangage acute heart failure from rapid AF. Again, this is unusual unless the heart rate is higher than 150. A reasonable approach then would be to ctrl rate with amiodarone or magnesium, both less likely to cause worsening of the heart failure, like apparently happened. Another treatment option would have been to cardiovert immediately.
When the patient was in extreme hypotensive shock, a cardioversion was appropriately done, even though the heart rate at that time was only 110-120, even less likely to be the primary cause of the hypotension. To cardiovert, a low dose of propofol was given. I don´t really see a reason to use the sedative most likely to cause hypotension in this case, a more appropriate choice would have been to use ketamine or ethomidate.
It sounds like what really saved the patient was the dobutamine drip.
Well I agree that propofol should be used cautiously in cardiac patients but I definitely wouldn’t have used ketamine here, the vasospasm risk is way scarier…. As for etomidage I do use it sometile but I’ve never really found it enough to sedate the patient
Thanks for a good review. It appears that this patient´s life was saved, but as discussed in the post, maybe not using the most ideal approach to CHF pulmonary edema.
One of the first rules to consider with tachycardia is to determine if the patient is ill from the tachycardia, or tachycardic because he/she is ill. Usually this is determined by the cut-off 150 bmp. Someone who is below 150, like this patient was, is unlikely to be ill primarily from the tachycardia.
The symptoms, signs and CXR reported in this case all point to CHF pulmonary edema with fluid overload as the patients primary condition. A reasonable approach might have been to give diuretics, nitrate and CPAP and observe if the tachycardia improved.
Another thing to discuss here is that the rhythm appears to have been determined from the 12 lead EKG only. The 12 lead is only a snapshot, ideal to determine axis, intervals and morphology. To determine rhythm in complex cases you need to keep an eye on the monitor for longer periods, perhaps try vagal manouvers and often you can then see a brief period of 3:1 flutter or other diagnostic clues. When reviewing the EKG, it certainly is most consistent with AF. In V1 there are however suspiciously regular waves that could be consistent with P-waves. I would suggest to reivew the monitor here.
Apart from tho focus of the post, how to manage acute CHF, a side topic of this is how we mangage acute heart failure from rapid AF. Again, this is unusual unless the heart rate is higher than 150. A reasonable approach then would be to ctrl rate with amiodarone or magnesium, both less likely to cause worsening of the heart failure, like apparently happened. Another treatment option would have been to cardiovert immediately.
When the patient was in extreme hypotensive shock, a cardioversion was appropriately done, even though the heart rate at that time was only 110-120, even less likely to be the primary cause of the hypotension. To cardiovert, a low dose of propofol was given. I don´t really see a reason to use the sedative most likely to cause hypotension in this case, a more appropriate choice would have been to use ketamine or ethomidate.
It sounds like what really saved the patient was the dobutamine drip.
Such patient in my workplace would have received cordarone and if unsuccesful cardioverted…. We were taught never to give ß-blockers in AHF….
It doesn’t make a difference in management, but this ECG shows atrial flutter, not fib. That said, it is a very unusual AV conduction: normally, the QRS occurs at exactly the same place on each flutter wave. Thus, whether conduction is 1:1, 2:1, 3:2, etc, the RR interval should be an integer multiple the wave interval. Thus, atrial flutter may be irregular, but should be “regularly” irregular. Here there is irregular AV conduction and so it makes the flutter irregularly irregular, mimicking atrial fibrillation. You can clearly see very regular flutter waves (upright “P’s”) in V1.
excellent review
Looks like there is a relatively new study on diuretics in the ED.
This may change our practice again.
Matsue Y, Damman K, Voors AA, et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol 2017; 69:3042.
Thanks for the comment and reading! This is a prospective, observational cohort including 1291 patients from multiple centers in Japan, with patients split to “early” (< 1 hour) and "late" (> 1 hour) for furosemide treatment. The early group receiving furosemide demonstrated unadjusted mortality of 2.3%, and the late group 6%. There are several issues with the study, as patients in the early group were more like to arrive via EMS and have greater pulmonary congestion, HR, and BP. Patients in the later group likely presented a greater diagnostic challenge for clinicians, potentially delaying diagnosis and treatment. Patients with delayed diagnosis and treatment do probably experience higher mortality. However, this study highlights something Ryan Radecki points out: “This probably turns the heart of the comparison into “appropriately recognized” and “possibly mismanaged”, rather than a narrow comparison of simply furosemide, early vs. not.” See here for more: http://www.emlitofnote.com/?p=3939
Rather than ensuring patients receive furosemide in a dedicated time (yet another metric to meet for us in the ED), it’s probably of greater importance to ensure accurate diagnosis. Adequate diuresis is important in heart failure, but accurate diagnosis is important as well.