Elevated Liver Enzymes: ED-focused Management Pearls & Pitfalls
- Jul 17th, 2016
- Eric Sulava
- categories:
Authors: Eric Sulava, MD (EM Resident at Naval Medical Center Portsmouth) and Samuel Bergin, MD (EM Resident at University Medical Center of Southern Nevada) // Edited by: Alex Koyfman, MD (@EMHighAK, EM Attending Physician, UTSW / Parkland Memorial Hospital) and Brit Long, MD (@long_brit)
Case
An 18 year-old female presents at 6 am with nausea and non-bloody, non-bilious emesis. Her mother reports that the patient just went through a recent break up with her boyfriend. The patient admits that last night she took some ‘painkillers’ at 11pm. She has vomited 3-4 times at home. Her mother identifies the pills as a previously unopened 30-count bottle of 500mg acetaminophen – 9 tablets remain.
Exam reveals vital signs: temperature 37.9C, pulse 103, respiratory rate 17, blood pressure 114/76, and weight 50kg. She is awake, alert, and quietly answering all questions appropriately. She appears disheveled and concerned, but with goal directed speech and appropriate demeanor. HEENT, cardiac, and pulmonary exams do not reveal any abnormalities. Her abdominal exam is notable for minimal right upper quadrant tenderness, but is otherwise normal. She is now denying any current suicidal ideation.
What labs should be drawn in this case? Would the liver function tests (LFTs) show abnormalities 7 hours following ingestion, and if so, in which pattern? Should activated charcoal or any other medications be given before or after acetaminophen levels are drawn?
Introduction
In today’s medical system, marginal laboratory values can lead to expensive, unnecessary, and potentially harmful further diagnostic evaluations. A 2012 retrospective, multi-center cohort study of patients presenting to the ED showed that laboratory testing has a direct effect on patients’ emergency department (ED) length of stay, with an average increase of 10 minutes for every five individual tests ordered (1). With routine incorporation of hepatic tests in blood chemical panels, it is crucial to have a detailed understanding of the pathophysiologic basis of liver function tests in order to establish appropriate clinical correlation and patient disposition.
Liver Biology: a little understanding goes a long way
The liver is a complex organ with multiple roles. Hepatocytes are organized into primary functioning units called the liver acinus, each of which is bordered by the ‘portal triad’ consisting of a branch of the hepatic artery, portal vein, and bile duct. This organization allows the liver to complete a wide array of tasks: glucose storage, glycogen breakdown, carbohydrate – fat – protein metabolism, bile synthesis, lipoprotein – plasma protein synthesis, detoxification, and waste product metabolism. All of these interacting roles can be impacted during liver disease, with corresponding alteration in liver function tests.
An LFT panel typically includes aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), prothrombin time (PT), and albumin. Clinically, LFT results are described in a ‘hepatocellular’ or ‘cholestatic’ arrangement based on the pattern of elevation. Tables 1 and 2 display the different LFTs and causes of elevation. Out of the most commonly ordered liver chemistry tests, true measurement of synthetic hepatic function is only assessed by prothrombin time (PT) and albumin. A hepatocellular pattern depicts a disproportionate elevation in the transaminases in comparison to ALP. The inverse, a disproportionate elevation of ALP in comparison to the transaminases, represents a cholestatic pattern. Serum bilirubin can be elevated in either pattern, and may indicate an extrahepatic disorder. Table 1 summarizes the commonly associated disorders that cause hepatocellular and cholestatic patterned abnormalities.
Aminotransferases are used to monitor and detect the progression of hepatocellular injury. Aspartate aminotransferase (AST) and serum alanine aminotransferase (ALT) are abundant hepatic enzymes crucial to citric acid cycle function. ALT and AST are released from damaged hepatocytes after hepatocellular injury or death. Both aminotransferases are highly concentrated in the liver, but ALT is generally considered to be more specific to liver damage; AST is amply expressed in the brain, skeletal muscle, kidney, and heart. Aminotransferase levels vary based on age and sex, so institutional reference limits should be specifically defined (3).
Alkaline phosphatase is an enzyme that transports metabolites across cell membranes. Liver and bone disease are the most common causes of pathological elevation, though other sites of origin include the intestines, kidney, and placenta (4). Due to the high body tissue prevalence, non-pathologic processes, like pregnancy, can cause elevation in laboratory values. During pregnancy, ALP begins to rise by the late first trimester and can reach twice normal values by term (5). In the liver, ALP is present in the bile duct epithelial cells; thus, biliary stasis can increase the release of the enzyme (6). Given the overlap in values with liver and bone disease, GGT can be used to differentiate the location of release. GGT is a glycoprotein located on the membranes of cells with high secretory or absorptive activities and is not abundant in bone.
Initial evaluation
When evaluating a patient with abnormal liver biochemical tests, the initial focus will be identifying potential risk factors of liver disease. The past medical history should be focused on conditions with associated hepatobiliary disease as seen in Table 3 (2, 7-10). The social and medication history should assess for potential hepatotoxins, including medications, drugs, and alcohol. Other rare methods of hepatotoxin exposure include recreational mushroom picking (Amanita phalloides) and occupational exposure to chemicals like vinyl chloride. The patient should be directly questioned on over the counter medications, herbal remedies, dietary supplements, and illicit drug use. The type and route of illicit drug should be identified, noting that IV drug use is a risk factor for viral hepatitis. Other risk factors for viral hepatitis include blood transfusion prior to 1992, travel to endemic hepatitis areas, and exposure to patients with jaundice (4).
During the evaluation a standard head to toe examination should be completed, focusing on the abdomen. In cases of chronic liver disease stigmata of cirrhosis can be seen: spider nevi, palmar erythema, gynecomastia, and caput medusae. Other common features of alcoholic cirrhosis include Dupuytren’s contractures (persistent digit flexion), parotid gland enlargement, and testicular atrophy (11). The abdominal exam characterizes the size and consistency of the liver. An enlarged tender liver could be due to acute viral hepatitis, while an enlarged, hard, nodular liver would represent a possible malignancy. Further palpation would reveal right upper quadrant tenderness with associated Murphy’s sign, suggesting cholestatic disease. The major physical exam findings of each syndrome will be explained in detail below.
Differential based on Magnitude of Transaminase Elevation
The National Health and Nutrition Examination Survey evaluated 6800 American patients and found elevated liver transaminase levels in up to 8.9% of the survey population (12). With this high of a prevalence, an algorithmic approach can aid in evaluation. Both the magnitude and ratio of AST and ALT can aid in narrowing the spectrum of disease. True “reference ranges” for these enzymes vary between establishments, and therefore will be addressed by magnitudes elevated above normal. Overall magnitude of aminotransferase elevation can be used to guide an initial diagnosis by categorizing the disorder based off of mild (<5x), moderate (5-10x), or marked elevation (>10x).
Mild Transaminase Elevation
1. Non-Alcoholic Fatty Liver Disease (NAFLD)
Nonalcoholic fatty liver disease is the most common cause of mildly altered liver enzyme levels in the western world, with a point-prevalence of 23% among American adults (13). Of note, it is a diagnosis of exclusion. NAFLD is a continuum, ranging from benign steatosis to steatohepatitis and cirrhosis. The fatty liver change occurs secondary to metabolic syndrome: obesity, diabetes mellitus, hypertension, and dyslipidemia (14). NAFLD is found in a bimodal distribution of age – it is the most common liver abnormality of children ages 2-19 (15). This patient demographic usually presents with vague complaints and has an incidental finding of transaminase elevations. The transaminase levels rarely exceed 4x the upper limit of normal with an AST/ALT ratio of < 2. Other conditions should be ruled out with history, exam, and laboratory testing. Treatment of this disorder is going to be completed through outpatient follow up. Lifestyle modification with weight loss, blood sugar control, and reduction of cholesterol is the mainstay of therapy.
2. Drug Induced Liver Injury
Idiosyncratic drug-induced liver injury (DILI) covers a wide spectrum of disease and accounts for 13% of acute liver failure cases in the U.S. (16). DILI is a broad term applied to any injury to the liver by a prescribed medication, over-the-counter medication, herb, or dietary supplement. The National Institutes of Health maintains a searchable database of over 1000 DILI associated substances (17). A 2008 study on 300 DILI patients in the United States listed Amoxicillin/clavulanate (8%), Nitrofurantoin (4%), Isoniazid (4%), and Trimethoprim-sulfamethoxazole (4%) as the most common inducing agents, excluding acetaminophen (18). Table 4 includes other commonly reported medications (19). Statins are frequently associated with elevated transaminase levels in new onset users; however, recent data shows that increases in transaminase levels have not been proven significantly different than those in patients taking placebo, and that elevated transaminase levels spontaneously resolve in up to 70% of persons taking statins, even with continued use (20,21).
Diagnostically approaching a patient with DILI is difficult due to the vast array of possible sources and clinical presentation. An algorithm developed by the Mayo Clinic is included below, as Table 5 (22). A list of all medications, herbals, and OTC substances should be obtained. As step 2 shows, it is important to determine if DILI is the primary diagnosis or simply a correlating factor to disease. Other common causes of liver dysfunction (viral hepatitis, alcohol related, EBV, NAFLD, and biliary obstruction) must be considered. Removal of the offending substance is the mainstay of treatment. All non-essential medications should be removed, following a risk-benefit discussion with the patient. Depending on severity, supportive care with 2-4 week follow up labs in the primary care setting is appropriate (22). A small subset of DILI patients present with elevated levels of gamma-globulins, antinuclear, and/or anti-smooth muscle antibodies. These patients are classified as having drug-induced autoimmune-like hepatitis (DI-AIH), which is responsive to prednisone therapy; this will likely be identified outside the ER setting (23).
3. Alcohol Induced Liver Injury
The liver is the main site of alcohol metabolism and a major target organ of alcohol-induced injury. There are three basic forms of alcohol related liver disease: alcoholic fatty liver disease, alcoholic cirrhosis, and alcoholic hepatitis, discussed below. Of note, alcoholic hepatitis can cause moderate LFT elevation. Fatty liver disease and cirrhosis are chronic issues due to a long history of alcohol use. The clinical manifestations of long-standing alcohol disease depend on its severity and chronicity.
Fatty liver disease is typically asymptomatic, while cirrhosis may have the classic findings of ascites, spider angiomata, gynecomastia, and palmar erythema. Diagnostic evaluation for patients with a strong history of chronic alcohol abuse will include CBC, LFT (serum transaminases, bilirubin, alkaline phosphatase, GGT, albumin), coagulation studies, and a complete chemistry panel. If other risk taking behaviors are reported (e.g. unprotected sex and IV drug use) viral hepatitis panels would be appropriate. The characteristic biochemical results includes an AST/ALT ratio greater than 2, with elevated GGT. The AST elevation is usually less than 8x the upper limit of normal, and the ALT elevation is typically less than 5x the upper limit of normal, categorizing chronic alcohol disease as mild to moderate transaminase elevation (2). Hematologic findings in patients with alcoholic liver disease may include thrombocytopenia with a macrocytic anemia. Longstanding alcoholics are at risk for poor nutrition with electrolyte and vitamin abnormalities, including vitamin B12, B1 and folate deficiency. The mainstay of treatment for chronic alcohol abuse will be the recommendation of abstinence from alcohol. Acute treatment in the ER will include vitamin, nutrient, and electrolyte repletion (24). Due to the synergistically destructive effect of chronic alcohol disease and thiamine deficiency, these patients are at risk for Wernicke-Korsakoff Syndrome. Supplementation with an IV bag of fluid containing thiamine, folic acid, and magnesium sulfate is recommended (25). Depending on the history, these patients are at risk for acute alcohol withdrawal, which should be considered.
Moderate Transaminase Elevation
1. Alcoholic Hepatitis
A 2015 US study on over 550,000 hospitalizations demonstrated the rate of alcoholic hepatitis related hospitalizations has significantly increased over the last decade, accounting for significant morbidity, mortality, and financial burden (26). Although the term ‘alcoholic hepatitis’ represents a spectrum of disease, ranging from asymptomatic steatohepatitis to acute hepatic encephalopathy, it is typically used to describe the acute onset of alcohol induced jaundice, anorexia, fever, and tender hepatomegaly. Patients with AH are usually 40-50 years old, with risk factors including female gender, Hispanic ethnicity, drinking multiple types of alcohol, drinking alcohol between mealtimes, poor nutrition, and obesity (27, 28). As with all alcohol related liver disease, the amount of alcohol consumption which places an individual at risk of developing alcoholic hepatitis is not known, but most patients with alcoholic hepatitis drink more than 100 g ⁄ day (29). Patients often stop drinking as they become ill, so it is common for patients to decrease their alcohol intake several weeks prior to presentation. Due to the acute hepatic inflammation, physical exam will show hepatomegaly with right upper quadrant tenderness. An audible hepatic bruit has been reported in a minority of patients. AH can be an acute complication to chronic cirrhosis, and therefore patients may present with stigmata of chronic liver disease (28).
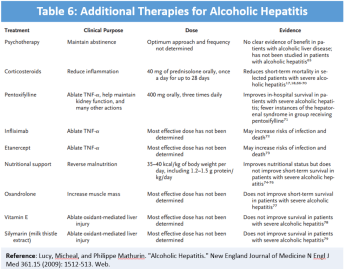
Patients with AH typically present with moderate elevations of AST/ALT (less than 300un/L), AST:ALT >2, elevated serum bilirubin, elevated GGT, moderate leukocytosis (neutrophils), and elevated INR (30). The above mentioned clinical and laboratory features are often adequate to secure the diagnosis of AH in a patient with a long history of heavy alcohol use, following appropriate rule out of mimicking disorders. The differential diagnosis for AH is extensive, including DILI, acute viral hepatitis, ischemic hepatitis, NASH, and chronic liver disease. Initial workup with an acute viral hepatitis panel, consisting of Anti-hepatitis A IgM, Hepatitis B surface antigen, anti-hepatitis B core IgM, and Anti-hepatitis C virus (HCV) antibodies is warranted, along with a transabdominal ultrasound (Doppler) to evaluate for viral hepatitis, biliary obstruction, and Budd Chiari syndrome. If the diagnosis is uncertain, then a liver biopsy can establish the diagnosis with greater certainty. Understandably, although generally recommended, a majority of these interventions are unrealistic in emergent care. Supportive care will be the primary focus of an ED provider, with the above-mentioned diagnostics organized by an admitting team or outpatient provider. Supportive care in acute episodes of AH includes alcohol abstinence, prevention and treatment of alcohol withdrawal, fluid management, nutritional support, infection surveillance, and PPI prophylaxis. Various calculators have been developed to determine disease severity and additional therapy. The Maddrey discriminant function score is a calculation using PT and serum bilirubin that estimates overall mortality risk. Patients with a score over 32 [DF= (4.6 x (PT – control PT)) + serum bilirubin] have high short-term mortality and may benefit from glucocorticoid therapy (31). Typical course is prednisone 40mg for 28 days, followed by an appropriate taper. Admitting teams may discuss Pentoxifylline, a TNF inhibitor, which has been suggested as replacement therapy for glucocorticoids. This has overall weak supporting data and currently has little utility in the ED. Table 6, a table from a NEJM review of alcoholic hepatitis, shows the other therapy options for acute AH. Most are not indicated due to risk for increased mortality or lack of overall benefit (30).
2. Biliary Tract Disease
The third National Health and Nutrition Examination Survey estimated that in the U.S., 6.3 million men and 14.2 million women had gallbladder disease, making it a very common primary complaint in the ER setting (32). Gallstones can cause significant colicky pain, biliary obstruction, or ascending biliary infection depending on their size and location. Risk factors for biliary disease include age over 40, female sex, number of pregnancies, obesity, family history, diabetes mellitus, cirrhosis, hemolysis, and medications (33). Medications commonly involved in biliary sludging and biliary disorders include estrogen, oral contraceptives, octreotide, clofibrate, and ceftriaxone (34). Patients with uncomplicated gallstone disease typically present with right upper quadrant (RUQ) pain, which radiates to the scapula and worsens with eating. A lodged stone can lead to biliary obstruction and cholangitis, presenting with Charcot’s triad of fever, jaundice, and RUQ pain. If left untreated, cholangitis can cause sepsis and altered mental status, making up Reynold’s pentad. Acute cholecystitis will show a moderate to severe increase of the transaminases depending on the severity of the disease. As disease progresses to choledocholithiasis and cholangitis, patients will produce elevated serum total bilirubin, indirect bilirubin, ALP, GGT, and at times leukocytosis (4). Confirmation of the diagnosis requires additional imagining. RUQ ultrasound may reveal gallstones, thickened gallbladder wall, sonographic Murphy’s sign, or dilated common bile duct. If the RUQ ultrasound is inconclusive, a hepatoiminodiacetic acid scan (HIDA) is indicated, but unfortunately often impractical for use in the ED setting. In this test technetium labeled HIDA is taken up by the hepatocytes and excreted into the bowel. A normal study will have visualization of contrast in the gallbladder, common bile duct, and small intestine by 30-60 minutes. Failure to do so identifies a blockage. Cholescintigraphy has a sensitivity and specificity for acute cholecystitis of approximately 90 to 97% and 71 to 90%, respectively (35).
ED management depends directly on the severity and type of biliary disease. Patients with uncomplicated biliary colic can be discharged with surgical follow up for an elective cholecystectomy, following pain control with NSAIDs or opioids. A commonly used NSAID is ketorolac, which is dosed at 30 – 60mg (IV/IM) depending on age and renal function. Symptomatic or complicated cholecystitis patients will likely require admission and should be placed NPO while receiving IV hydration, analgesia, and antiemetics. A surgical consultation is appropriate depending on clinical status. If choledocholithiasis is suspected, further diagnostics are needed. The admitting team will organize an ERCP or MRCP depending on the patient’s clinical status and risk. ERCP is both diagnostic and therapeutic, while MRCP is much less invasive. Due to serious complications of pancreatitis, bleeding, and perforation, ERCP is reserved for patients who are at high risk for having a common bile duct stone, particularly if there is evidence of cholangitis (36). As mentioned above, sepsis is an immediate concern for acute cholangitis patients. Hemodynamic stabilization and antibiotic treatment with a broad-spectrum agent should be initiated in the ED. Although there is no consensus for a superior regimen for biliary sepsis, broad coverage for gram negative and anaerobic organisms is generally recommended. Once admitted, antibiotic therapy can be modified to reflect blood culture results. Consultations for ERCP biliary drainage should be placed as soon as possible if available. Percutaneous transhepatic cholangiography (PTC) can be considered when ERCP is unavailable, unsuccessful, or contraindicated (37).
Severe Transaminase Elevation
-
Acute Viral Hepatitis
Although the incidence of hepatitis A, hepatitis B, and hepatitis C have all been declining over the past two decades, their clinical relevance remains, and they continue to be reportable diseases (38). While few patients will require hospitalization and exact determination of viral hepatitis is unlikely in the ED, early initiation of diagnosis is important. On initial evaluation serological hepatitis panels, transaminases (10-100x increase, with ALT>AST), and bilirubin (5-25 mg/dL) should be evaluated. The most common emergent interventions for patients with viral hepatitis will consist of fluid and electrolyte corrections due to diarrhea and vomiting. Some patients with altered mental status with reduction in hepatic synthetic function (INR above 1.5 or hypoglycemia) should be admitted and observed for possible fulminant hepatitis (39).
Hepatitis A (HAV), a fecal-oral RNA virus, has had a nationally routine childhood vaccine since 2005, with a corresponding decrease in incidence. The primary risk factors are now men who have sex with men, intravenous drug users, and international travelers (38,40,41). The symptoms of hepatitis A include a gradual development of fever, nausea, vomiting, diarrhea, abdominal pain, and occasional jaundice, following a 2-8 week incubation period. Clinical research has shown that only 10%-30% of HAV patients develop these symptoms, making clinical suspicion heavily historically based (41). Since the maximal viremia and infectivity are prior to onset of symptoms, HAV is diagnosed with an antibody test, Anti-HAV IgM (42). Treatment is primarily symptomatic, though immune serum globulin can help in post-exposure prophylaxis. Hand washing and proper food preparation are the primary defenses (41). Hepatitis E is similar to Hepatitis A in the aspects of transmission, being fecal-oral, and symptomatology. HEV, known for causing significant disease in pregnant women, lacks a widespread vaccine. Its geographical distribution is primarily limited to Asia, Africa, and Russia. The diagnosis of (HEV) is made via serum / stool PCR, or by the detection of IgM antibodies to HEV. Unfortunately there are no commercial tests for HEV licensed in the U.S., and CDC research labs must be contacted for testing (43). Ribavirin and pegylated interferon are being investigated as potential treatments for chronic HEV (44,45).
Hepatitis B is a profoundly infectious enveloped DNA virus that has highest incidence, like Hepatitis A, among men who have sex with men and with intravenous drug abuse. Age strongly determines likelihood of chronic carrier state with it occurring in only 10% of adults compared to 90% of neonates (46). The symptoms include: malaise, fever, nausea, vomiting, occasional arthralgia, and resolving jaundice (47). A notable difference is the absence of diarrhea in HBV. Diagnosis is established using HB-surface antigen, HB-core IgM (acute), HB-core IgG (chronic), and HB-envelope antigen (corresponds to infectivity). While the infection may be occult, it can occasionally lead to fulminant hepatitis, hepatic failure, and encephalopathy; this is most common in the presence of an HBV and HDV co-infection. Fulminant hepatitis is recognized via altered mentation and spontaneous mucosal bleeding (48). Post-exposure prophylaxis for unvaccinated and vaccinated (dependent on titer) patients may include one time HBIG and HBV vaccine. Hepatitis D is only found in patients who are actively infected with HBV, with a worldwide incidence of approximately 5% of chronic HBV carriers. Presence of HDV is suggestive of a more sinister disease course (49).
Hepatitis C is most common among intravenous drug users, HIV-positive patients, those with greater than 20 sexual partners, and persons who received blood transfusions prior to 1992 (50). Like other viral hepatitis causes, acute HCV infections are typically asymptomatic. However, unlike Hepatitis A & B, progression to chronic hepatitis with HCV is very common and approaches 90% of patients (51). Diagnosis is partially performed by elimination of other causes and can be confirmed via HCV enzyme immunoassay, PCR or ELISA. Classically treatment may include interferon alfa-2b (with or without polyethylene glycol) and ribavirin (52). There are now newer and very effective medications, ledipasvir and sofosbuvir, with treatment protocols dependent on the genotype (53). Post-exposure prophylaxis is unclear at this time.
2. Ischemic Injury
Severe transaminase elevations are due to global hepatocellular injury or necrosis, which can be caused by an acute hypoxic event. The liver’s complex vascular supply and high metabolic rate make it vulnerable to circulatory disturbances. Ischemic hepatitis, also referred to as ‘shock liver’, refers to severe hepatic injury secondary to acute hypoperfusion. This injury is not mediated by an inflammatory process, as the name hepatitis implies, but by hemodynamic instability. This includes global circulatory compromise from extrahepatic sources, like acute ACS, CHF, sepsis, and trauma. More focal causes of hepatic hypoperfusion include hepatic artery thrombosis, portal vein thrombosis, surgical ligation, or hepatic sickle cell crisis (54). The hemodynamic instability is usually apparent clinically before evidence of liver injury appears. Lab chemistries usually show a rapid rise in serum aminotransferases to >25X the upper limit of normal, followed by a dramatic increase in LDH. The transaminase levels will peak within three days of the initiating event, and in the absence of continued clinical decline, will normalize by days 7-10 (55). Managing ischemic hepatitis includes hemodynamic resuscitation, with close monitoring of end organ perfusion. After acute stabilization of the patients in the ED, immediate transfer to ICU level care should occur.
3. Acetaminophen Toxicity
Acetaminophen, the most widely used analgesic-antipyretic in the United States, is the most common cause of drug induced hepatic failure (4). The Annual Report of the American Association of Poison Control reported that in 2014 there were over 70,000 acetaminophen related overdoses, resulting in 107 deaths (56). The medication’s potency is partially due to its rapid and complete uptake in the intestinal tract, causing peak concentrations 1.5-2 hours following administration. When doses exceed the maximum daily recommendations of 80 mg/kg in children and 4 g in adults, acetaminophen begins to rapidly deplete hepatic glutathione stores via the overproduction of NAPQI by the cytochrome 450 pathway. When hepatic glutathione stores are depleted, NAPQI begins to cause irreversible oxidative hepatocyte injury and hepatocellular centrilobular necrosis. Patients who are chronic alcoholics have increased CYP2E1 activity, which further depletes glutathione levels, and puts them at increased risk for acetaminophen toxicity (57). Other medications that cause a similar reaction include anticonvulsants, antituberculosis drugs (isoniazid – rifampin), and Bactrim.
Clinical manifestations of acetaminophen overdose are often mild and nonspecific, but due to the importance of a proper ingestion timeline, a four stage clinical course has been developed. Stage 1 represents 0.5-24 hours after the overdose with nausea, vomiting, diaphoresis, pallor, and malaise. Progression to stage 2, 24-72 hours after ingestion, includes rapid transaminase elevation with symptomatic hepatic injury of RUQ pain, hepatic enlargement, and abdominal tenderness. LFT abnormalities peak during stage 3, representing 72-96 hours, during which time the severe complications of hepatic encephalopathy, hyperammonemia, and bleeding diathesis can occur. Death can occur in this stage from a combination of multiorgan failure and lactic acidosis (58). Stage 4 represents the recovery phase following the 96 hour timestamp.
For further evaluation a 4-hour post ingestion acetaminophen serum level should be drawn. If the ingestion was more than four hours ago it should be drawn immediately. If the time of ingestion is unknown, draw a lab immediately and then again in four hours for continued monitoring. The time of ingestion and acetaminophen serum level will be used with the Rumack-Matthew nomogram to determine the severity of acetaminophen toxicity and the need for treatment with N-acetylcysteine (NAC). NAC rapidly repletes glutathione stores, preventing toxic metabolite formation if given within 8 hours of ingestion. NAC was found to be safe for administration up to 24 hours following ingestion, noting the treatment was only effective if given within the first 8 hours (59). It can be given intravenously with a 20 hour protocol or orally with a 72 hour protocol. The oral regimen is the treatment of choice, if not otherwise contraindicated. FDA-approved dosage regimen for oral NAC starts with a loading dose of 140 mg/kg, followed by 17 doses, each at 70 mg/kg, given every 4 hours. Other treatment includes gastrointestinal decontamination with 1g/kg of activated charcoal, if within four hours of ingestion (60).
Conclusions
LFT are commonly ordered laboratory tests with a variety of abnormalities in a vast array of disorders. By understanding the biochemical basis of each test, it is possible to correlate laboratory findings to a patient’s clinical presentation. By separating common hepatic disorders into subcategories based on the magnification of transaminase elevation, a more simplistic algorithm like approach can be taken to help narrow the spectrum of a differential diagnosis. This can be used to help eliminate waste in costly and unnecessary follow up studies by maximizing the understanding of what an LFT result represents.
- LFTs = ‘hepatocellular’ or ‘cholestatic’ arrangement based on the pattern of elevation.
- Hepatocellular pattern = transaminases > ALP
- ALT is generally considered to be more specific to liver damage
- Past medical history and social history are crucial insight to hepatic risk factors (table 3)
- Magnitude of aminotransferase elevation => guide initial diagnosis: mild (<5x), moderate (5-10x), or marked elevation (>10x)
- Mild = NAFLD, Drug Induced Liver Injury, Alcohol Induced Liver Injury
- Moderate = Alcoholic Hepatitis, Biliary Tract Disease
- Severe = Acute Viral Hepatitis, Ischemic Injury, Acetaminophen Toxicity
- As always, supportive care is key in the ED!
References/Further Reading
- Li L, Georgiou A, Vecellio E, Eigenstetter A, George Toouli MPH (2015). The effect of laboratory testing on emergency department length of stay: A multihospital longitudinal study applying a cross classified random-effect modeling approach. Academic of Emergency Medicine 22: 38-46.
- Giannini, E.G., Testa, R., Savarino, V. Liver enzyme alteration: a guide for clinicians. CMAJ.2005;172:367–379.
- Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem 2000;46(12):2050-68.
- Fishman WH. Alkaline phosphatase isoenzymes: recent progress. Clin Biochem 1990;23(2):99-104
- Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology 2002;123(4):1367-84.
- Fishman WH, Bardawil WA, Habib HG, Anstiss CL, Green S. The placental isoenzymes of alkaline phosphatase in sera of normal pregnancy. Am J Clin Pathol 1972;57:65–74
- Moss DW. Physiochemical and pathophysiological factors in the release of membrane-bound alkaline phosphatase from cells. Clin Chim Acta 1997;257 (1):133-40.
- Gizard, E., A. C. Ford, J.-P. Bronowicki, and L. Peyrin-Biroulet. “Systematic Review: The Epidemiology of the Hepatobiliary Manifestations in Patients with Inflammatory Bowel Disease.” Alimentary Pharmacology & Therapeutics Aliment Pharmacol Ther 40.1 (2014): 3-15. Web.
- Younossi, Zobair. “Changes in the Prevalence of the Most Common Causes of Chronic Liver Diseases in the United States from 1988 to 2008.” SciVee (n.d.): n. pag. Web.
- Valdivieso, V. “Pregnancy and Cholelithiasis: Pathogenesis and Natural Course of Gallstones Diagnosed in Early Puerperium.” Hepatology 17.1 (1993): 1-4. Web.
- Ford, Ryan M., Wendy Book, and James R. Spivey. “Liver Disease Related to the Heart.” Transplantation Reviews29.1 (2015): 33-37. Web
- Rosen, Peter, John A. Marx, Robert S. Hockberger, Ron M. Walls, and James Adams. “Chapter 90: Disorders of the Liver and Biliary Tract. “Rosen’s Emergency Medicine: Concepts and Clinical Practice. 8th ed. Philadelphia, PA: Mosby Elsevier, 2006. 1186-204. Print.
- Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol. 2006;101(1):76-82.
- Harrison SA, Kadakia S, Lang KA, Schenker S. Nonalcoholic steatohepatitis: what we know in the new millennium. Am J Gastroenterol 2002;97(11):2714-24.
- Maheshwari A, Thuluvath PJ. Endocrine diseases and the liver. Clin Liver Dis. 2011;15:55–67.
- Pacifico L, Poggiogalle E, Cantisani V, Menichini G, Ricci P, Ferraro F, Chiesa C. Pediatric nonalcoholic fatty liver disease: a clinical and laboratory challenge. World J Hepatol. 2010;2(7):275–288.
- Lammert C, Einarsson S, Saha C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003–2007
- http://www.livertox.nih.gov/
- Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924-1934, 1934.e1-4
- Navarro, Victor J., and John R. Senior. “Drug-Related Hepatotoxicity.” New England Journal of Medicine N Engl J Med7 (2006): 731-39. Web
- Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C-60C.
- Bays H. Statin safety: an overview and assessment of the data—2005. Am J Cardiol. 2006;97(8A):6C-26C.
- Leise, Michael D., John J. Poterucha, and Jayant A. Talwalkar. “Drug-Induced Liver Injury.” Mayo Clinic Proceedings1 (2014): 95-106. Web.
- Czaja, Albert J. “Drug-Induced Autoimmune-Like Hepatitis.” Dig Dis Sci Digestive Diseases and Sciences4 (2011): 958-76. Web.
- Blendis, L. M. “Review Article: The Treatment of Alcoholic Liver Disease. “Alimentary Pharmacology & Therapeutics5 (2007): 541-48. Web. Flannery, A. H., and D. A. Adkins.
- “Unpeeling the Evidence for the Banana Bag: Evidence-Based Recommendations for the Management of Alcohol-Associated Vitamin and Electrolyte Deficiencies in the ICU.” Crit Care MedMarch (2015): n. pag. Web.
- Jinjuvadia, Raxitkumar, and Suthat Liangpunsakul. “Trends in Alcoholic Hepatitis-related Hospitalizations, Financial Burden, and Mortality in the United States.” Journal of Clinical Gastroenterology6 (July, 2015): 506-11. Web.
- Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis 2004; 24: 217–32
- Cohen, S. M., and J. Ahn. “Review Article: The Diagnosis and Management of Alcoholic Hepatitis.” Alimentary Pharmacology & Therapeutics1 (2009): 3-13. Web.
- Sohail, Umair, and Sanjaya K. Satapathy. “Diagnosis and Management of Alcoholic Hepatitis.” Clinics in Liver Disease4 (2012): 717-36. Web.
- Lucy, Michael, and Philippe Mathurin. “Alcoholic Hepatitis.” New England Journal of Medicine N Engl J Med15 (2009): 1512-513. Web.
- Maddrey WC, Boitnott JK, Bedine MS et al. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978; 75(2):193-199.
- Everhart, James E., Meena Khare, Michael Hill, and Kurt R. Maurer. “Prevalence and Ethnic Differences in Gallbladder Disease in the United States.” Gastroenterology3 (1999): 632-39. Web.
- Conte, Dario, Mirella Fraquelli, Fabio Fornari, Lucia Lodi, Paolo Bodini, and Luigi Buscarini. “Close Relation Between Cirrhosis and Gallstones.” Arch Intern Med Archives of Internal Medicine1 (1999): 49. Web.
- Shiffman, M. L., and F. B. Keith. “Pathogenesis of Ceftriaxone-associated Biliary Sludge. In Vitro Studies of Calcium-ceftriaxone Binding and Solubility.” 99.6 (1990): 1772. Web.
- Kiewiet, Jordy J. S., Marjolein M. N. Leeuwenburgh, Shandra Bipat, Patrick M. M. Bossuyt, Jaap Stoker, and Marja A. Boermeester. “A Systematic Review and Meta-Analysis of Diagnostic Performance of Imaging in Acute Cholecystitis.” Radiology3 (2012): 708-20. Web.
- Giljaca, Vanja, Kurinchi Selvan Gurusamy, Yemisi Takwoingi, David Higgie, Goran Poropat, Davor Štimac, and Brian R. Davidson. “Endoscopic Ultrasound versus Magnetic Resonance Cholangiopancreatography for Common Bile Duct Stones.” Cochrane Database of Systematic Reviews2 (2015)
- Bornman, Philippus C., Johan I. Van Beljon, and Jake Krige. “Management of Cholangitis.” Journal of Hepato-Biliary-Pancreatic Surgery6 (2003): 406-14. Web.
- Centers for Disease Control and Prevention (CDC): Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill SUMM 2009: 58: pp. 1-27
- Gotthardt, D., C. Riediger, K. H. Weiss, J. Encke, P. Schemmer, J. Schmidt, and P. Sauer. “Fulminant Hepatic Failure: Etiology and Indications for Liver Transplantation.” Nephrology Dialysis TransplantationSupplement 8 (2007): Viii5-iii8. Web.
- McNabb SJ, et al: Summary of notifiable disease—United States, 2005. MMWR Morb Mortal Wkly Rep 2007; 54: pp. 1
- Fiore AE, Wasley A, and Bell BP: Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55: pp. 1
- “Positive Test Results for Acute Hepatitis A Virus Infection Among Persons With No Recent History of Acute Hepatitis–United States, 2002-2004.” JAMA: The Journal of the American Medical Association8 (2005): 894-96. Web
- Takahashi, M., S. Kusakai, H. Mizuo, K. Suzuki, K. Fujimura, K. Masuko, Y. Sugai, T. Aikawa, T. Nishizawa, and H. Okamoto. “Simultaneous Detection of Immunoglobulin A (IgA) and IgM Antibodies against Hepatitis E Virus (HEV) Is Highly Specific for Diagnosis of Acute HEV Infection.” Journal of Clinical Microbiology1 (2005): 49-56. Web.
- Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A. “Ribavirin for chronic hepatitis E virus infection in transplant recipients” N Engl J Med.2014;370:1111–1120.
- Kamar, Nassim, Lionel Rostaing, Florence Abravanel, Cyril Garrouste, and Laure Esposito. “Pegylated Interferon‐α for Treating Chronic Hepatitis E Virus Infection after Liver Transplantation.” Clinical Infectious Diseases CLIN INFECT DIS5 (2010): n. pag. Web.
- Dienstag JL: Hepatitis B virus infection. N Engl J Med 2008; 359: pp. 1486-1500).
- Wasley A, Miller JT, and Finelli L: Surveillance for acute viral hepatitis—United States, 2005. MMWR Surveill Summ 2007; 56: pp.1
- Yurdaydin C, IDilman R, Bozkaya H, and Bozdayi AM: Natural history and treatment of chronic delta hepatitis. J Viral Hepat 2010; 17: pp. 749-756
- Romeo, Raffaella. “Hepatitis Delta Virus: Making the Point from Virus Isolation up to 2014.” WJH World Journal of Hepatology22 (2015): 2389. Web.
- Sears, Dawn M., Dan C. Cohen, Kimberly Ackerman, Jessica E. Ma, and Juhee Song. “Birth Cohort Screening for Chronic Hepatitis During Colonoscopy Appointments.” Am J Gastroenterol The American Journal of Gastroenterology6 (2013): 981-89. Web.
- Jaeckel E, et al: Treatment of acute hepatitis C with interferon alfa-2b. NEJM 2001; 345: pp. 1452-1457
- Swain, Mark G., Ming–Yang Lai, Mitchell L. Shiffman, W. Graham E. Cooksley, Stefan Zeuzem. “A Sustained Virologic Response Is Durable in Patients With Chronic Hepatitis C Treated With Peginterferon Alfa-2a and Ribavirin.” Gastroenterology5 (2010): 1593-601. Web.
- Chhatwal, Jagpreet, Fasiha Kanwal, Mark S. Roberts, and Michael A. Dunn. “Cost-Effectiveness and Budget Impact of Hepatitis C Virus Treatment With Sofosbuvir and Ledipasvir in the United States.” Annals of Internal Medicine Ann Intern Med6 (2015): 397. Web.
- Seeto, Reginald K., Ben Fenn, and Don C. Rockey. “Ischemic Hepatitis: Clinical Presentation and Pathogenesis.” The American Journal of Medicine2 (2000): 109-13. Web.
- Gitlin, N., and Km Serio. “Ischemic Hepatitis: Widening Horizons.” Am J Gastroenterol. 87.7.831 (1992):Web.
- Mowry JB, Spyker DA, Brooks DE, McMillan N, Schauben JL. 2014 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd Annual Report. Clin Toxicol (Phila). 2015 Dec. 53 (10):962-1147.
- Zimmerman, Hyman J., and Willis C. Maddrey. “Acetaminophen (paracetamol) Hepatotoxicity with Regular Intake of Alcohol: Analysis of Instances of Therapeutic Misadventure.” Hepatology3 (1995): 767-73. Web.
- Bessems, Jos G. M., and Nico P. E. Vermeulen. “Paracetamol (Acetaminophen)-Induced Toxicity: Molecular and Biochemical Mechanisms, Analogues and Protective Approaches.” Critical Reviews in Toxicology1 (2001): 55-138. Web.
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988 Dec 15. 319(24):1557-62.
- Brok, Jesper, Nick Buckley, and Christian Gluud. “Interventions for Paracetamol (acetaminophen) Overdose. “Cochrane Database of Systematic Reviews(2006): n. pag. Web






Elevated AST or ALT is present in only about 40% of cases of cholecystitis according to a prominent JAMA review article (http://jama.jamanetwork.com/article.aspx?articleid=195707)