Pediatric Small Talk – The Rule of 6: Pediatric Abdominal Surgical Emergencies
- Nov 3rd, 2021
- Nessy Dahan
- categories:
Welcome back to Small Talk. Every first Wednesday of the month we will release high yield PEM content written by PEM talent from around the country. We hope you enjoy these reviews. Comments, questions, accolades or concerns: feel free to reach out to Joe Ravera, MD (pemgemspod@gmail.com).

Authors: Nessy Dahan, MD and Brittany Francisco, DO (Pediatric Emergency Medicine Fellow, NewYork-Presbyterian Brooklyn Methodist Hospital, Department of Emergency Medicine, Division of Pediatric Emergency Medicine) // Reviewed by: Joe Ravera MD (@pemuvm1, Director of Pediatric Emergency Medicine, Assistant Professor of Surgery, Division of Emergency Medicine, University of Vermont Medical Center); Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)
Case:
A 15-month-old male with no reported past medical history presents to the pediatric emergency department (PED) with fussiness of 2-day duration. The patient has intermittent episodes of fussiness during the past two day, during which he pulls his knees up to his chest. Initially, these episodes lasted for a few minutes at a time, and the patient was otherwise playful and interactive. As the days progressed though, he became more lethargic, has not been tolerating PO, and has had decreased wet diapers as per baseline. The mother also reports that the patient has had multiple episodes of non-bloody, non-bilious emesis over the past two days. She denies any fever, diarrhea, bloody stools, respiratory distress, recent travel or sick contacts. The patient is up-to-date with his vaccinations.
The patient’s vital signs include T 37.2℃, HR 165 BPM, BP 70/50 mm Hg, RR 20, and 100% SpO2 on room air. The examination is notable for a lethargic and irritable appearing infant, tachycardia without murmurs, a diffusely tender abdomen to palpation, and a capillary refill of 3-seconds. Of note, you inspect the diaper where you notice a bloody-appearing stool.
Presentations of Abdominal Pain in Children:
Similar to the adult population, the differential diagnosis for a child with abdominal pain is broad. Depending on the history and exam, the list may include, though is not limited to, infectious (viral gastroenteritis or food-borne illnesses), genitourinary (cystitis or testicular/ovarian torsion), psychosomatic (anxiety), respiratory (pneumonia or asthma), cardiac (heart failure), gastrointestinal (inflammatory bowel disease, irritable bowel syndrome, or gastroesophageal reflux disease), endocrinological (diabetic ketoacidosis), oncological, (intracranial masses), neurologic (abdominal migraines) or abdominal surgical emergencies.1
While the list above may be extensive, missing surgical disease and delay in diagnosis is associated with major morbidity and mortality and as such needs to be considered in all cases of pediatric abdominal pain, vomiting, and a few other unusual presentations. One way to help remember the general ages of presentation for common pediatric abdominal surgical emergencies is the mnemonic known as “the rule of 6.”2 While not everyone follows the rule, common ages of presentation include: malrotation with midgut volvulus at around 6 days of life, pyloric stenosis at around 6 weeks of life, intussusception at around 6 months of life, and appendicitis at around 6 years of life.
The 6-Day-Old: Malrotation with Midgut Volvulus
Intestinal malrotation is a congenital anomaly usually occurring in the first trimester during the formation of the intestines. In utero, the bowels herniate through the umbilicus and undergo rotation for the anatomically correct bowel position. In malrotation, the bowel does not complete the full 270 degree rotation which leads to a short mesentery.3 Most commonly the cecum is on the left and the small intestine to the right of the superior mesenteric artery (Figure 1). Malrotation occurs in approximately 1 in 6,000 live births.3 Risk for volvulus, the twisting of intestines onto itself, is greatly increased in infants with malrotation. It is also associated with other abnormalities such as omphalocele, gastroschisis, jejunal atresia, and biliary atresia.
Figure 1: Normal and malrotated bowel anatomy. Image courtesy of Healthjade.net.

About 50% of cases present within the 1st month of life and about 90% present within the 1st year of life.1 Older children usually present with vague symptoms, such as nonspecific abdominal pain that can be acute or intermittently chronic, with or without nausea, vomiting, and diarrhea.4
Classically, malrotation with volvulus presents in infants with bilious emesis with abdominal distention and irritability. Although only about there are other causes of bilious vomiting in the neonatal period given the high mortality and morbidity of delay in diagnosis of management, it is important to remember that neonatal bilious vomiting is malrotation with volvulus until proven otherwise. Bloody stools and lethargy are late findings as indicators of necrotic bowel and are often associated with hemodynamic instability which can occur quickly in these infants. In these cases, aggressive fluid resuscitation, antibiotics, and emergent surgical consultation are recommended.
Current standard for definitive diagnosis is an upper GI series, which classically will show a “bird’s beak” of the 2nd or 3rd portion of the duodenum or a “corkscrew” appearance if duodenum is partially obstructed (Figure 2).5 An upper GI series is diagnostic even without obstruction because it will show the abnormal position of the duodeno-jejunal flexure (ligament of Treitz). Some clinicians will start the work-up with an abdomen x-ray, which may be normal or show nonspecific signs of obstruction.6 Recently, there was a large review that examined abdominal ultrasound as a diagnostic modality and demonstrated both high sensitivity and specificity. Ultrasonographic findings include the whirlpool sign and the reversal of SMA-SMV relationship, where the SMA is on the right and SMV on the left (Figure 3).7 An abdominal CT scan is usually used in older children where the presentation can be vague. CT imaging can show the whirlpool sign of mesentery, malrotation of bowel, obstruction, free air, and the reversal of SMA/SMV relationship (Figure 4).
Figure 2: Corkscrew sign seen in a patient with malrotation with midgut volvulus. Image courtesy of Radiopaedia.org.

Figure 3: Reversal of SMA-SMV seen on ultrasound in a patient with malrotation with midgut volvulus. Image courtesy of Radiopaedia.org.

Figure 4: Whirlpool sign seen on CT in a patient with malrotation with midgut volvulus. Image courtesy of Radiopaedia.org.
After initial stabilization with fluids, an emergent Ladd procedure must be performed by surgery. The surgeon will untwist the bowel and resect nonviable bowel segments prior to widening the mesentery to alleviate the obstruction by lysing the band between the cecum and lateral abdominal wall.
The 6-Week-Old: Pyloric Stenosis
Infantile hypertrophic pyloric stenosis, also known as pyloric stenosis, is the most common surgical cause of emesis in infants.8 The medical condition occurs as the pylorus becomes pathologically thickened, leading to obstruction of gastric outflow and subsequent emesis. Disease onset is typically between 5 to 6 weeks of life, and the disorder rarely occurs after twelve weeks of life.8,9 Although it should be noted that with the wide availability of ultrasound this diagnosis is sometimes made very early including week 2 or 3 of life. Its incidence is 1 in 250 births, with a 4:1 male to female ratio, and it has been seen more commonly in first born males.1 While the presentation and management of pyloric stenosis has been well-defined, its cause has remained unclear.8
Infants with pyloric stenosis typically present with projectile, non-bilious emesis after feeding. These infants are typically “hungry vomiters” as they exhibit wanting to feed immediately after the emesis occurs.1,9 Although rare to see, a triad of projectile emesis, visible peristalsis after feeding, and a palpable “olive” in the right upper quadrant, which is the hypertrophic pyloric muscle, has been described for patients with pyloric stenosis.9 A recent study has shown that a palpable ”olive” is seen only in 13.6% of cases, possibly due to earlier presentation and diagnosis of the disease process.11Despite this, a palpable “olive” on exam has been noted to have a 99% positive predictive value.8 These infants may initially be well-appearing and well-hydrated, though as the symptoms progress they may develop signs of dehydration such as sunken fontanelle, decreased urine output, and dry mucous membranes. Classically, patients may display a hypochloremic, hypokalemic metabolic alkalosis.10 This electrolyte imbalance is seen less frequently than the past per a recent study, with hypochloremia and alkalosis being present in 23% and 14.4% of patients, respectively, likely due to earlier diagnosis.11
The standard imaging choice for diagnosis of pyloric stenosis is ultrasonography. Although operator dependent, ultrasound’s accuracy for diagnosis is near 100%.12 The normal pyloric muscle wall thickness is less than 3mm, and the normal pyloric channel length is less than 14 mm.10,12 Abnormal increases in these measurements, commonly remembered by the mnemonic pi (π), is diagnostic for pyloric stenosis. (Figure 5 and 6). Other sonographic signs include the antral nipple sign, denoting “redundant pyloric mucosa that protrudes into the gastric antrum” (Figure 7), and the target sign, described as “hypertrophied hypoechoic muscle surrounding echogenic mucosa” (Figure 8).13 If the ultrasound is non-diagnostic, an upper GI series with barium can be performed if considering other causes of forceful emesis, such as malrotation, antral web, and gastroesophageal reflux.1
Figure 5: Pyloric channel length of 2.81 cm, concerning for pyloric stenosis. Image courtesy of Radiopedia.org.

Figure 6: Pyloric muscle wall thickness of 0.833 cm, concerning for pyloric stenosis. Image courtesy of Radiopedia.org.

Figure 7: Antral nipple sign seen in pyloric stenosis. Image courtesy of Radiopedia.org.

Figure 8: Target sign seen in pyloric stenosis. Image courtesy of Radiopedia.org.

Management of patients with pyloric stenosis begins with correction of electrolyte abnormalities with intravenous fluid rehydration. Surgical consultation should be obtained for curative surgical correction with a pyloromyotomy 8-10. The prognosis for these patients is excellent, and feeding may begin 4-8 hours after surgical correction.10 After surgery, infants are admitted for hospital observation and are discharged after proving that they can tolerate PO feeds.1,10
The 6-Month-Old: Intussusception
Intussusception is an emergent abdominal condition in which a segment of bowel invaginates and telescopes into an adjacent distal segment of bowel.1,9 Commonly seen between the age of 6 to 36 months, it is the most common cause of intestinal obstruction in infants and toddlers.9 The peak age of incidence is 5 to 9 months of age14, with 80% of cases occurring before age 2.9 It is more predominantly seen in males in a 3:28 to a 2:114 ratio. Ninety percent of cases are idiopathic and caused by lymphoid hyperplasia in Peyer’s patches associated with a prior viral infection. Ten percent of cases are secondary to a pathological entity that causes a lead point, which promotes the formation of the intussusception. These underlying pathologies include intestinal polyps, mesenteric nodes, Henoch-Schönlein purpura, duplication cysts, Meckel’s diverticulum, and cystic fibrosis.9,14 Majority of cases are ileocolic in location1, though ileoileal intussusception cases have been seen, most commonly in Henoch-Schönlein purpura.15 The index of suspicion for a pathologic lead point is raised in children who present outside the typical age range or have recurrent intussusception.
A child with intussusception typically presents with intermittent episodes of colicky abdominal pain, in between which the patient is well-appearing and at his or her baseline. Some children may also have bilious emesis or a palpable mass in the right upper quadrant (RUQ). As the disease process advances, a late finding of “currant jelly” stools may be present due to bowel ischemia with mucosal sloughing.14 A reported triad of abdominal pain, currant jelly stools, and palpable RUQ mass is only seen in <15% of cases.9
In addition to abdominal symptoms, children with intussusception can also present with neurologic symptoms. One review reported 17% of children with intussusception presented with neurologic symptoms with the most common being lethargy and hypotonia.15 Given the waxing and waning nature of symptoms a high index of suspicion is required when evaluating a toddler with altered mental status as the GI symptoms may not be present at time of evaluation.
Abdominal ultrasonography has become the initial test of choice for diagnosis, with reported sensitivities ranging from 98-100%1,14 and specificities of 88-100%.14 Intussusception can be identified via the target sign (Figure 9), which refers to a sonodense center surrounded by a sonolucent ring in the transverse plane, representing bowel contents surrounded by bowel wall.12 The intussuscepted bowel may also have the appearance of a pseudokidney in the longitudinal plane (Figure 10), referred to as the pseudokidney sign.12,17 Ileocolic intussusception may be differentiated from its ileoileal counterpart by the diameter of the pathological bowel, with a diameter >2 cm favoring the former.17 Abdominal x-rays may be used for diagnosis, and may show signs of bowel obstruction and/or an elongated mass in the RUQ.1,17
Figure 9: Target sign seen on ultrasound in a patient with intussusception. Image courtesy of Radiopedia.org

Figure 10: Pseudokidney sign seen on ultrasound in a patient with intussusception. Image courtesy of Radiopedia.org

Management of intussusception should begin with keeping the patient NPO and providing IV hydration, especially if clinically dehydrated. Reduction of the intussuscepted bowel is typically achieved via air or contrast enemas, with success rates noted to be 80-85%.17 The choice of contrast type is typical institution dependent. Of note, the risk of recurrence after enema has been seen in up to 10% of cases.9 If reduction is not achieved via enema, or if the patient has signs of intestinal perforation before or during the procedure, surgical correction may be necessary.1
The 6-Year-Old Appendicitis:
Acute appendicitis is the most common surgical emergency in children.1 Appendicitis is caused by the obstruction of the appendix by a fecalith/appendicolith or lymphoid hyperplasia, leading to bacterial overgrowth causing inflammation and dilation of appendix. Peak incidence is 9 to 12 years of age and rarely is seen children < 2 years of age.1 Perforated appendicitis can occur within 24 hours of symptoms and is common after 72 hours of symptoms.1 Appendicitis should be considered in any child with abdominal pain without prior appendectomy.
Children usually present with periumbilical pain with anorexia at the beginning of presentation. Fever, nausea with or without emesis start to occur and then pain moves to the classic right lower quadrant (RLQ). Localization of pain depends on the child and the location of the appendix; for example, a retrocecal appendicitis may have pain with hip flexion or back pain.18 Tenderness in the RLQ at McBurney’s point is typical for appendicitis. Guarding and rebound tenderness are also classically seen with appendicitis. Another physical exam finding commonly seen is Rovsing’s sign where a patient has RLQ pain when the left lower quadrant is palpated.
Evaluation for appendicitis consists of lab work, fluids, pain control and imaging. Usually, a child will have an elevated white blood cell (WBC) count within the first day of illness, and the WBC will continue to rise as the appendix becomes more gangrenous. However, a normal WBC does not exclude appendicitis.1,9,18
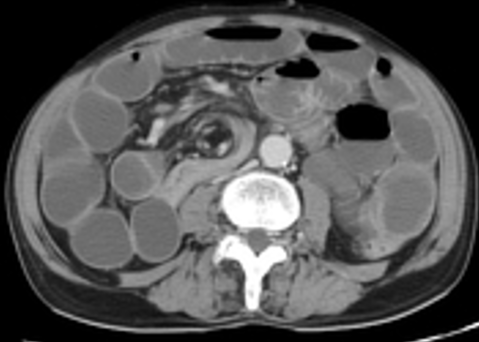
Imaging is helpful and should be acquired if the history and physical are suspicious for appendicitis. While abdominal X-ray is unlikely to give the diagnosis, it can be helpful to determine free air in perforation and may show an appendicolith (Figure 11). Especially in the pediatric population, ultrasound is the imaging modality of choice because of its lack of ionizing radiation. Ultrasound findings supportive of appendicitis include a non-compressible, dilated (> 6mm) appendix (Figure 12), with a hyperechoic appendicolith (Figure 13), and secondary signs of inflammation such as echogenic fat surrounding the appendix.1 To confirm the structure is the appendix the sonographer must demonstrate the structure to be blind-ending and originating from the cecum. If the appendix cannot be visualized on ultrasound then an abdominal CT scan would be the next step. CT imaging is highly sensitive and specific but does expose the child to radiation. The CT can show appendiceal dilatation (> 6mm) (Figure 14), wall thickening, and periappendiceal inflammation and necrosis.
Figure 11: Appendicolith seen in the right lower quadrant. Image courtesy of Radiopaedia.org.

Figure 12: Dilated (8mm) appendix indicating appendicitis. Image courtesy of Radiopaedia.org.

Figure 13: Appendix with appendicolith. Image courtesy of Radiopaedia.org.

Figure 14: Appendiceal dilation with appendicolith. Image courtesy of Radiopaedia.org.

After initial stabilization, surgery should be consulted for an appendectomy. If caught early, the prognosis for these patients is very good after surgical correction. Complications of appendicitis can include perforation and abdominal abscesses, which both may prolong the patient’s hospitalization course and outcome.1,9,18
Mimickers:
While one should be vigilant when evaluating pediatric patients with emesis and/or abdominal pain to rule out the above surgical processes, there are several mimicking medical conditions that should be kept on the differential diagnosis list. GERD is commonly encountered as a cause of emesis in neonates. Viral gastroenteritis, food-borne illnesses, and mesenteric adenitis may mimic symptoms of appendicitis, though examination may help distinguish between which child to pursue further work-up. Although a diagnosis of exclusion, constipation is a frequent cause of abdominal pain in toddlers and school age children. A lower lobe pneumonia may lead to a young patient having fever, abdominal pain, and emesis. Inflammatory bowel disease, whether Crohn’s disease or ulcerative colitis, may lend to gastrointestinal and extraintestinal symptoms. Special attention should be paid to the possibility of urological or gynecological sources of abdominal pain, such as testicular or ovarian torsion, pregnancy, incarcerated hernia, tubo-ovarian abscess, or pelvic inflammatory disease. One should perform a genitourinary exam, especially in a male pediatric patient with abdominal pain, to rule out a possibility of testicular torsion, as this condition may present initially with vague, non-specific abdominal pain.
Case Conclusion:
Given the patient’s appearance and presentation, you obtain IV access, administer a normal saline 20 cc/kg bolus and antibiotics, obtain lab-work, and set up a point-of-care abdominal ultrasound that reveals a characteristic “target sign,” concerning for intussusception. You contact pediatric surgery and radiology, and the patient is taken to the radiology suite, where he has successful reduction after a contrast enema.
Pearls:
- Think of “the rule of 6” to help remember the general ages for common pediatric abdominal surgical emergencies.
- ~ 6 days old: Malrotation with volvulus
- ~ 6 weeks old: Pyloric Stenosis
- ~ 6 months old: Intussusception
- ~ 6 years old: Appendicitis
- Neonatal bilious vomiting is malrotation and volvulus until proven otherwise and an upper GI series is a good standard for diagnosis of malrotation regardless of if there is obstruction at the time of presentation.
- The diagnostic standard for pyloric stenosis is ultrasound, with “pi” being a mnemonic to remember the normal pyloric muscle wall thickness and channel length measurements (3 and 14 mm, respectively).
- An infant presenting with altered mental status and/or shock may have an underlying diagnosis of intussusception.
- Consider appendicitis as a possible diagnosis in any child with abdominal pain without a history of an appendectomy.
Pitfalls:
- Remember to examine the genitourinary region of male pediatric patients with abdominal pain, as testicular torsion can present with non-specific abdominal pain.
- Don’t forget that lower lobe pneumonia may present with fever, abdominal pain and emesis, especially in the younger population.
- While most children present with malrotation before 1 year of age, be concerned for malrotation with volvulus in older children who have intermittent, chronic abdominal pain and bilious emesis.
- Although the classic electrolyte imbalance of hypochloremic, hypokalemic metabolic alkalosis is seen less often now due to earlier diagnosis of pyloric stenosis, remain vigilant in rehydration and electrolyte repletion for dehydrated infants with this disorder.
- Most patients with intussusception do not present with the triad of abdominal pain, currant jelly stools, and palpable abdominal mass. Consider intussusception in the toddler with colicky abdominal pain.
- A normal WBC does not exclude appendicitis. Use your clinical gestalt to help guide the management of children with abdominal pain concerning for possible appendicitis.
References:
- Shaw KN, Bachur, RG, et al., Fleisher & Ludwig’s Textbook of Pediatric Emergency Medicine, 2016.
- Ravera, Joe. “Pediatric Abdominal Surgical Disease: The Rule of 6” PEM GEMS. www.pemgems.com/rule-of-6/.
- Alani M, Rentea R, “Midgut Malrotation” StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK560888/
- Nehra D, Goldstein AM, Intestinal malrotation: varied clinical presentation from infancy through adulthood, Surgery. 2011 Mar;149(3):386-93.
- Fox S, “Malrotation” Pediatric EM Morsels https://pedemmorsels.com/malrotation/.
- Coste A, Anand S, Nada H, Ahmad H, Rentea R, “Midgut Volvulus” StatPearls.
- Zhang W, Sun H, Luo F. The efficiency of sonography in diagnosing volvulus in neonates with suspected intestinal malrotation. Medicine (Baltimore). 2017 Oct;96(42):e8287
- Ranells JD, Carver JD, Kirby RS. Infantile hypertrophic pyloric stenosis: epidemiology, genetics, and clinical update. Adv Pediatr. 2011;58(1):195-206.
- Macias CG, et al. Strange and Schafermeyer’s Pediatric Emergency Medicine, 5E. The McGraw-Hill Companies, Inc, 2019.
- Garfield K, Sergent SR. Pyloric Stenosis. [Updated 2021 Feb 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555931/
- Glatstein M, Carbell G, Boddu SK, Bernardini A, Scolnik D. The changing clinical presentation of hypertrophic pyloric stenosis: the experience of a large, tertiary care pediatric hospital. Clin Pediatr (Phila). 2011 Mar;50(3):192-5. doi: 10.1177/0009922810384846. Epub 2010 Nov 22. PMID: 21098534.
- Ma, O. John. Ma and Mateer’s Emergency Ultrasound. McGraw-Hill Education, 2021.
- Amini, Behrang. “Pyloric Stenosis: Radiology Reference Article.” Radiopaedia Blog RSS, radiopaedia.org/articles/pyloric-stenosis?lang=us
- Waseem, M., & Rosenberg, H. K. (2008). Intussusception. Pediatric Emergency Care, 24(11), 793–800. doi:10.1097/pec.0b013e31818c2a3e
- Kleizen KJ, Hunck A, Wijnen MH, Draaisma JM. Neurological symptoms in children with intussusception. Acta Paediatr. 2009 Nov;98(11):1822-4.
- Weiss PF. Pediatric vasculitis. Pediatr Clin North Am. 2012 Apr;59(2):407-23. doi: 10.1016/j.pcl.2012.03.013. Epub 2012 Apr 6. PMID: 22560577; PMCID: PMC3348547.
- Amini, Behrang. “Intussusception: Radiology Reference Article.” Radiopaedia Blog RSS, radiopaedia.org/articles/intussusception?lang=us.
- Bundy DG, Byerley JS, Liles EA, Perrin EM, Katznelson J, Rice HE, Does this child have appendicitis?, JAMA 2007 Jul; 298(4):438-51

thank you very much