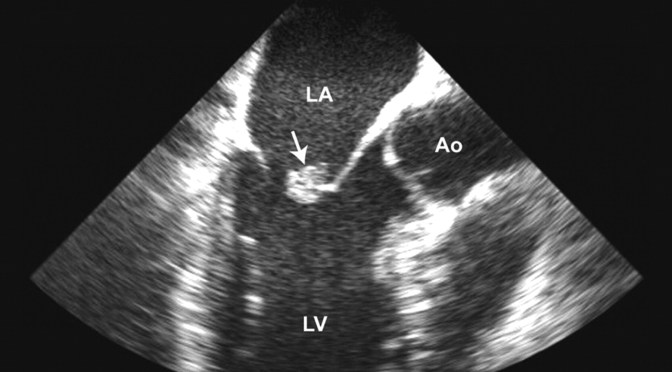
A Case of Non-bacterial Thromboembolic Endocarditis
A 59 year-old male presented to the emergency department with a chief complaint of difficulty concentrating and loss of vision. He had presented to the same facility the day prior for chest pain, chills, and a cough. During his prior visit, the patient underwent a chest x-ray which demonstrated a consolidation suggestive of a lobar pneumonia and was subsequently discharged home with a prescription for Azithromycin as well as instructions to follow-up with his primary care doctor. However, he was unable to fill his prescription. Upon attempting to drive home, the patient was pulled over by law enforcement because he was acting “delirious.” Despite the traffic incident, he was allowed to return home. The patient reported that once he arrived at home he began bumping into furniture, experiencing difficulty with concentration, and suffered vision loss. In addition, he continued to experience chills, chest pain, and shortness of breath.
A Case of Non-bacterial Thromboembolic Endocarditis Read More »