Authors: Taylor Goller, MD (EM Resident Physician, Virginia Tech-Carilion Emergency Medicine); Sarah Klemencic, MD (EM Attending Physician, Virginia Tech-Carilion Emergency Medicine Residency) // Reviewed by: Alexander Y. Sheng, MD, MHPE (@TheShenger); Alex Koyfman, MD (@EMHighAK); Brit Long (@long_brit)
Cases
#1: A 21-year-old female presents with mild left flank pain and dysuria. She is a member of the cross-country running team and competed in her conference championship two days ago. Her dipstick urinalysis shows bacteriuria, pyuria, leukocyte esterase (LE)+, nitrate +, and large blood. In addition to treating her urinary tract infection (UTI), what other conditions must be considered and what follow up should be recommended?
#2: A 72-year-old male presents with visible blood in his urine for two days. He notes that the urine becomes pink towards the end of voiding and he has noted occasional clots. He denies dysuria, hesitancy, or incontinence. He has a history of hypertension, prostate cancer status post prostatectomy with radiation therapy, and atrial fibrillation on warfarin. He worked as a painter for 30 years and has a 50 pack-year smoking history. What dangerous etiologies must be considered and what follow up should be recommended?
Introduction
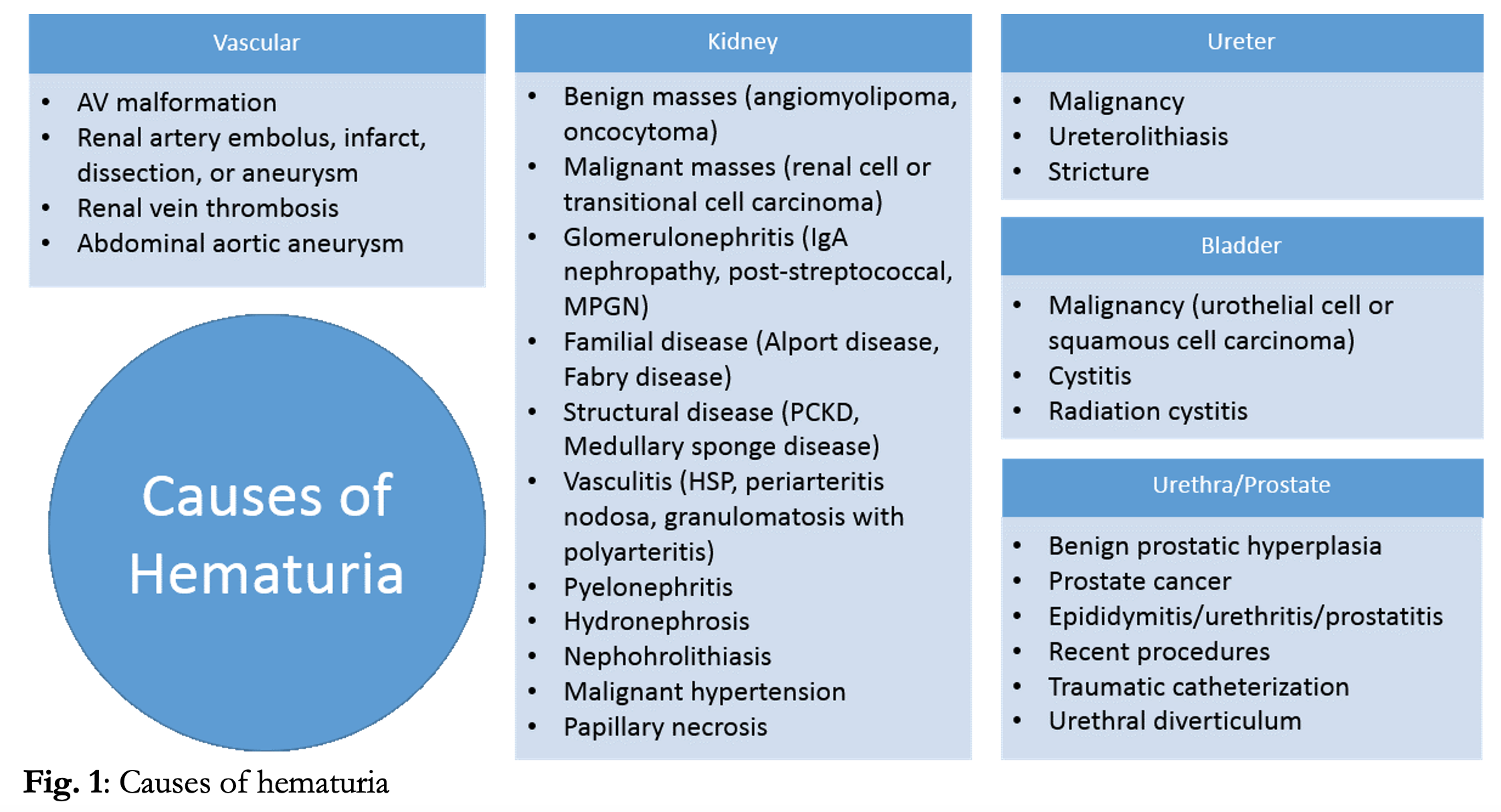
Hematuria is a common presentation in the emergency department, accounting for ~400,000 ED visits annually in the US.1Additionally, incidental hematuria may be noted during work-up of many other presenting complaints requiring urinalysis. Blood in the urine can be benign, and 13-40% of asymptomatic adults may demonstrate hematuria on urinalysis.2,3,4 However, the potential causes of hematuria are extensive (Fig. 1) and hematuria can be a marker of serious underlying disease including infection, nephropathy, malignancy and vascular pathology. The responsibility of the ED provider is to determine which cases can be managed conservatively, which require more extensive immediate work up and management, and which require referral to a specialist.
Discussion of traumatic hematuria is not the scope of this article. Please refer to the following post for details:http://www.emdocs.net/genitourinary-trauma-presentations-evaluation-and-management-updates/.
This article focuses on the workup and management of the patient with nontraumatic hematuria in the ED.
Urinalysis
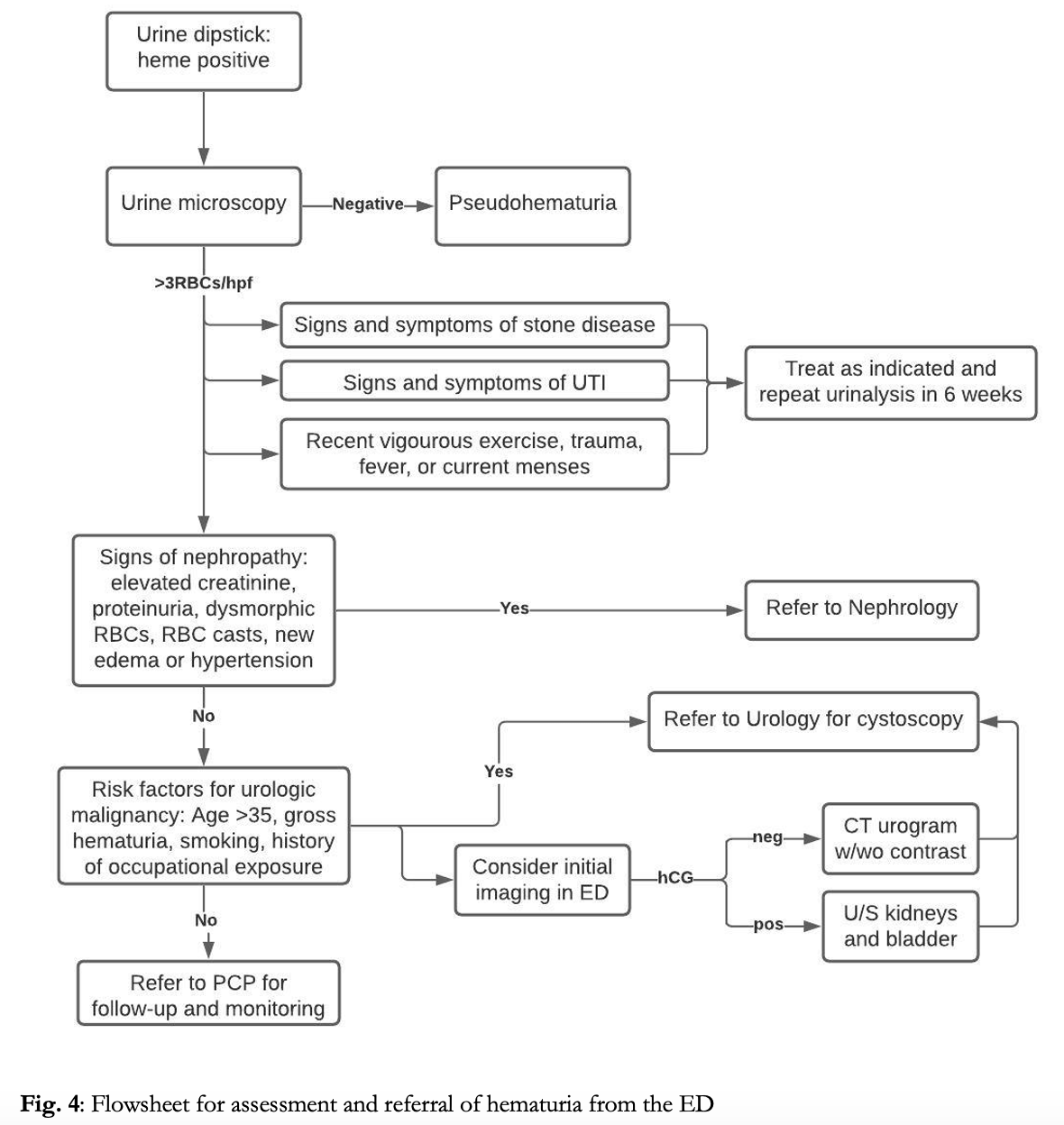
The urine dipstick is a useful first step to confirm the presence of blood. Healthy individuals may have up to 7 RBCs/mL of urine. Urine dipstick is able to detect the presence of 10 RBCs/mL and thus exhibits high sensitivity for physiologic abnormal hematuria.5False negative results can occur with high specific gravity, low pH, and high consumption of vitamin C.4,5 Discrete dots on the urine dipstick itself may indicate intact RBCs, while diffuse color change may indicate higher concentrations of RBCs, intravascular hemolysis, or the presence of myoglobin.5 False positives are most commonly caused by myoglobin from rhabdomyolysis or by intravascular hemolysis releasing free hemoglobin.4 All positive dipstick tests should be sent for microscopic analysis to determine true presence of red blood cells. In women, it is also necessary to consider contamination from the genital tract such as during menstruation. Pelvic exam, catheter sample, or collection of sample after placement of tampon and perineal cleaning may be useful to differentiate the true source of bleeding.

Pseudohematuria
Many different drugs, foods, and medical conditions can cause a red color to the urine often mistaken for blood, termed pseudohematuria. Causes of pseudohematuria are shown in figure 3.

Microscopic Hematuria
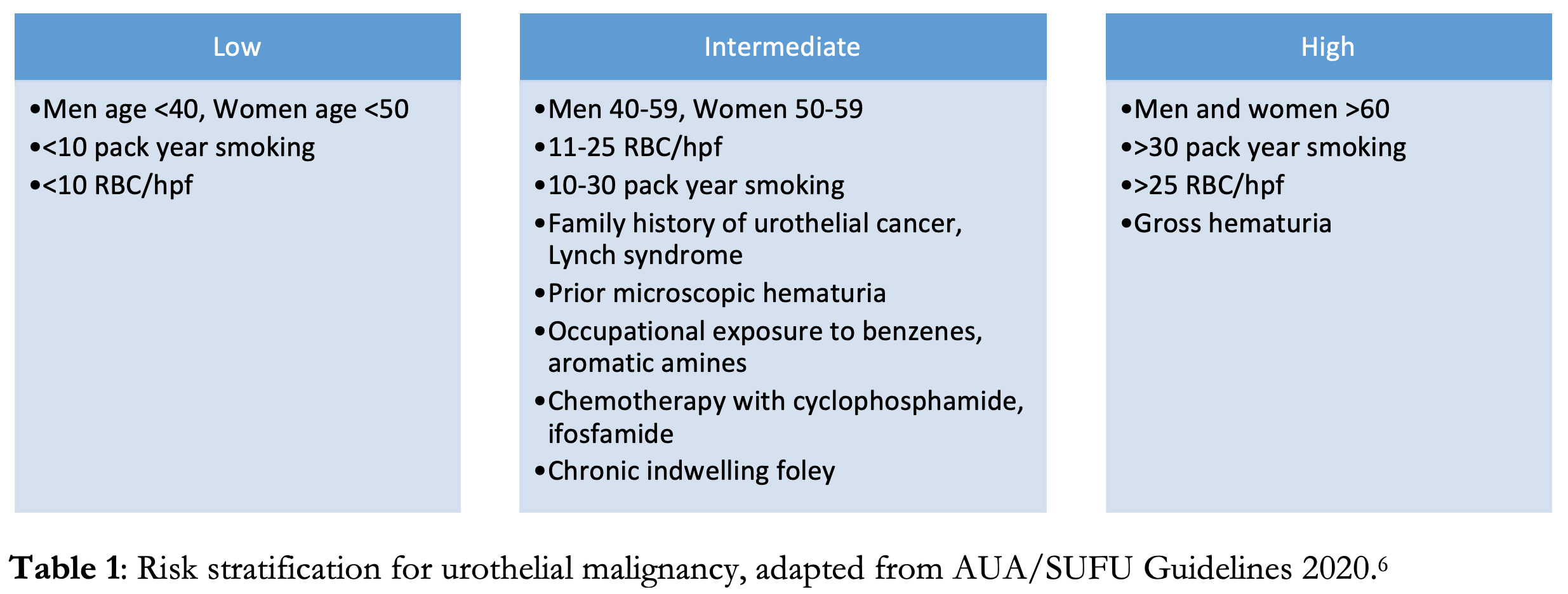
Microscopic hematuria is defined as ≥3-5 RBCs/hpf.5,6,7 Asymptomatic microscopic hematuria is most commonly benign, with serious urologic disease occurring in only ~2% of patients and renal or bladder carcinoma in <1%.3 It can often be seen in young individuals after vigorous exercise, sexual intercourse or with febrile illness.4 Elevated INR may lead to microscopic hematuria, however this rarely occurs in the therapeutic range. Additionally, 15% of urine samples obtained by urinary catheterization may demonstrate microscopic hematuria from the procedure itself, though rarely exceeding 3 RBCs/hpf.4 However, the differential for microscopic hematuria is broad, and serious life-threatening causes including malignancy, aortic abdominal aneurysm, or renal artery dissection should be considered, especially in patients with risk factors. The risk of malignancy is increased with age ≥35, male gender, occupational exposure to aromatic amines (commonly in hair dyes, paints and plastics) or history of smoking or chronic UTIs.6,7 The current American Urological Association (AUA) clinical practice guidelines recommend urological referral for all patients with microscopic hematuria in which a benign cause has been ruled out, and cystoscopy for all patients aged ≥35 or with current or past tobacco use or exposure to caustic chemicals.7
Macroscopic Hematuria
Macroscopic hematuria is defined by noticeable color change in the urine, which generally requires >10 RBCs/hpf or approximately 1 ml of blood. Gross visible hematuria, is more likely an indicator of underlying pathology. The incidence of malignancy with gross hematuria is 20-40%, and painless gross hematuria is the presenting symptom in 60% of urologic malignancies.7,8 The vast majority of urinary tract malignancies are urothelial (formerly transitional cell) carcinoma, though renal cell carcinoma can also present with gross hematuria and is more common in patients with a predisposing syndrome, such von Hippel-Lindau. Greater than 90% of urothelial cancers arise from the bladder.9 A single episode of gross hematuria is concerning and should prompt referral to urology even if symptoms have resolved.6,8 Additionally even in the setting of UTI or anticoagulation gross hematuria should be considered abnormal and prompt further work-up or referral.8
History and Physical Exam
Initial evaluation should focus on hemodynamic instability that could accompany sepsis or catastrophic vascular etiology. In the asymptomatic patient, recent exercise, sexual activity, menstruation, or fever, which can all produce transient benign microscopic hematuria.4 History should also focus on associated pain that could be associated with infection or stone disease. Recent upper respiratory infection could be associated with nephropathy. The presence of clots, and color of the urine during urination should be discerned. Dark urine that becomes lighter midstream or the presence of clots indicates a lower urinary tract source.8 Associated pain is common with vascular etiologies and stone disease, while painless hematuria is more common with bladder malignancy.4 Physical exam often adds little to differentiate cause. Flank tenderness can be associated with stones or infection. Cardiac murmur, abdominal mass or bruit, could indicate a vascular etiology. Peripheral edema can raise suspicion for a glomerulonephropathy. Suprapubic tenderness may be associated with obstruction.4
Labs and Imaging
Apart from urinalysis with microscopic evaluation and hCG in women of child bearing age, labs or imaging are not required by for microscopic hematuria. Workup for gross hematuria requires blood work with CBC, renal function tests, and coagulation studies in patients with risk factors for bleeding diathesis, and type and screen if concern for significant blood loss.
No broad consensus exists regarding initial imaging. And imaging does not need to be routinely performed for evaluation of hematuria in the ED. Imaging should be reserved to identify emergent diagnoses such as differentiating pyelonephritis from nephrolithiasis, or to rule out vascular pathology.4 The AUA recommends referral for CT urogram and cystoscopy for any episode of hematuria that falls in the high risk category.6 CT has a near 100% sensitivity for detecting malignancy of the upper urinary tracts and with proper excretory phase a 95% NPV for tumors of the bladder. However, in intermediate risk patients and in pregnancy, renal ultrasound is a reasonable initial imaging modality, which has 82% sensitivity for renal tumors, but is limited in its evaluation of more distal structures.6,9 MRI is a reasonable alternative for patients in which CT is unable to be performed, however with lower sensitivity than CT. While plain films and retrograde urethrograms have minimal utility in detecting underlying malignancy.6,9 In cases where timely follow up and referral is unavailable, advance imaging such as ultrasound or CT urography can be obtained in the ED to expedite the workup in patients at intermediate or high risk.
Pediatrics
In the pediatric population the prevalence of hematuria is 4-6%, and more common in females.2 Neonates produce benign urate crystals which are red in color, and often mistaken by concerned parents as hematuria.7 Gross hematuria is most commonly from urethrorrhagia (15% of cases), UTI (14%), trauma (14%), or congenital urinary tract anomalies (14%).2 Malignancy is rare except in cases of predisposing genetic syndromes, and children rarely require cystoscopy. However, a higher consideration should be given to inborn abnormalities such as polycystic kidney disease, and nephropathies. Glomerular disease is the most common cause of hematuria in children.4 Microscopic hematuria associated with increased urine protein, cellular casts, dysmorphic cells, or hypertension are indicative an underlying nephropathy.4,9 Recent sore throat or febrile illness should raise the possibility of post-streptococcal glomerulonephritis, which can be investigated with ASO titers and C3 levels. Associated proteinuria, urine:serum protein ratio >0.2, or RBC casts are suggestive of glomerular pathology commonly seen with Henoch-Schonlein purpura or IgA nephropathy. Any concern for nephropathy should prompt referral to nephrology.
Treatment
Without significant renal dysfunction, anemia, or other conditions that would otherwise require admission, most patients can be safely discharged with follow up. Discharge instructions should encourage fluid intake, frequent voiding and return precautions for darkening urine, urinary retention increasing pain, or fever.8 Patients with suprapubic pain or difficulty voiding should be evaluated for an obstructing lesion by imaging. In cases in which an obstructing clot is suspected, a three-way foley catheter should be placed with continuous irrigation until the outflow is clear.5,8 After irrigation, patients who are able to void spontaneously or with a catheter may be discharged with close follow up. Placement of a suprapubic catheter should be avoided to prevent seeding of the abdominal wall with urothelial malignancy.8 Infusion of TXA during irrigation has been shown to reduce the amount of irrigation needed and decrease hematuria detected by dipstick, however has not been shown to reduce the need for transfusion in serious GU bleeding.10Reversal of anticoagulation is rarely indicated except in cases of life-threatening hemorrhage or vascular etiology.4
Case Conclusions
Case 1: The patient’s urine was sent for urine microscopy, which confirmed hematuria with 7 RBCs/hpf. With her left flank pain a renal ultrasound was performed in the ED which did not demonstrate hydronephrosis. A CBC, BMP, and hCG were unremarkable, including a normal creatinine. She had a mildly elevated creatinine kinase. She was treated according to the hospital’s local antibiogram for pyelonephritis, given return precautions for worsening pain, gross hematuria, or signs of infection, and recommended to follow up in 6 weeks, after the conclusion of her cross country season, with her primary care provider for a repeat urinalysis to ensure resolution of her hematuria in the setting of UTI and strenuous exercise.
Case 2: The patient’s urine was sent for urinalysis and found to have >30 RBCs/hpf. A CBC and CMP were unremarkable, though the patient’s INR was supratherapeutic at 4.5. However, the patient was hemodynamically stable with no significant drop in Hgb. Further, a post void residual showed <50ml of retained urine. The significance of the bleeding was emphasized to the patient and he was referred to follow up with urology for a CT urogram and cystoscopy within 2 weeks. Pt was instructed to hold his warfarin for two days with clinic recheck. Return precautions were given for signs of worsening urinary retention, infection, or symptomatic anemia.
Key Points
-Hematuria should be confirmed by urine dipstick and microscopy to rule out pseudohematuria.
-All patients with hematuria older than 35, current or past tobacco use, or exposure to aromatic amines (commonly in hair dyes, paints and plastics) should be referred to urology for cystoscopy to rule out urologic malignancy.
-Adult patients with painless gross hematuria should get labs and imaging, including UA, CBC, Cr, BUN, and CT urogram. Advanced imaging can be deferred to the outpatient setting if timely follow up can be obtained.
-Hematuria with associated proteinuria, hypertension, or RBC casts should raise concern for an associated nephropathy.
-Pediatric patients rarely have urologic malignancies, but are at higher risk for congenital abnormalities and post-infectious nephropathies.
References
- National Hospital Ambulatory Medical Care Survey: 2017 Emergency Department Summary Tables. Centers for Disease Control and Prevention. 2017. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf
- Bignall ONR 2nd, Dixon BP. Management of Hematuria in Children. Curr Treat Options Pediatr. 2018;4(3):333-349. doi:10.1007/s40746-018-0134-z
- Mohr DN, Offord KP, Owen RA, Melton LJ 3rd. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA. 1986;256(2):224-229.
- Willis GC, Tewelde SZ. The Approach to the Patient with Hematuria. Emerg Med Clin North Am. 2019;37(4):755-769. doi:10.1016/j.emc.2019.07.011
- Roberts, James R., Catherine B. Custalow, Todd W. Thomsen, and Jerris R. Hedges. 2014. Roberts and Hedges’ clinical procedures in emergency medicine. 1444-46.
- Barocas D, Boorjian S, Alvarez R, et al. Microhematuria AUA/SUFU Guideline. American Urologic Association, 2020.
- Walls RM, Rosen P. (2018). Rosen’s emergency medicine: Concepts and clinical practice (9th ed.). Philadelphia, PA: Elsevier/Saunders. p1230-31, 2172-73.
- Hicks D, Li CY. Management of macroscopic haematuria in the emergency department. Emerg Med J. 2007;24(6):385-390. doi:10.1136/emj.2006.042457
- Dulku G, Shivananda A, Chakera A, Mendelson R, Hayne D. Painless Visible Haematuria in Adults: An Algorithmic Approach Guiding Management. Cureus. 2019;11(11):e6140. Published 2019 Nov 13. doi:10.7759/cureus.6140
- Moharamzadeh P, Ojaghihaghighi S, Amjadi M, Rahmani F, Farjamnia A. Effect of tranexamic acid on gross hematuria: A pilot randomized clinical trial study. Am J Emerg Med. 2017;35(12):1922-1925. doi:10.1016/j.ajem.2017.09.012
- Kurz M, Feldman AS, Parezella, MA. Etiology and evaluation of hematuria in adults. In: UpToDate, Lam AQ (Ed), UpToDate, Waltham, MA, 2018








