Welcome back to the “52 in 52” series. This collection of posts features recently published must-know articles. This week the series covers hydrocortisone in patients with severe community-acquired pneumonia, or the CAPE-COD trial.

Author: Brannon Inman (Chief Resident, Emergency Medicine Physician, San Antonio, TX) // Reviewed by: Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)
Hydrocortisone in Severe Community-Acquired Pneumonia
AKA: The “CAPE-COD” Trial
Clinical question:
In patients with severe community acquired pneumonia (PNA), does hydrocortisone reduce mortality at 28 days?
Study design:
- Double-blind, randomized controlled clinical superiority trial
- Authors expected that 1146 patients would result in an 80% power to detect a 25% mortality reduction.
- Enrolled 800 patients around the time of the COVID pandemic. The authors turned their attention to hydrocortisone for COVID-19 PNA in a dedicated embedded trial. These patients’ data were not included in the results of this trial. After the second interim analysis the data safety monitoring board recommended the trial be stopped due to perceived benefit.
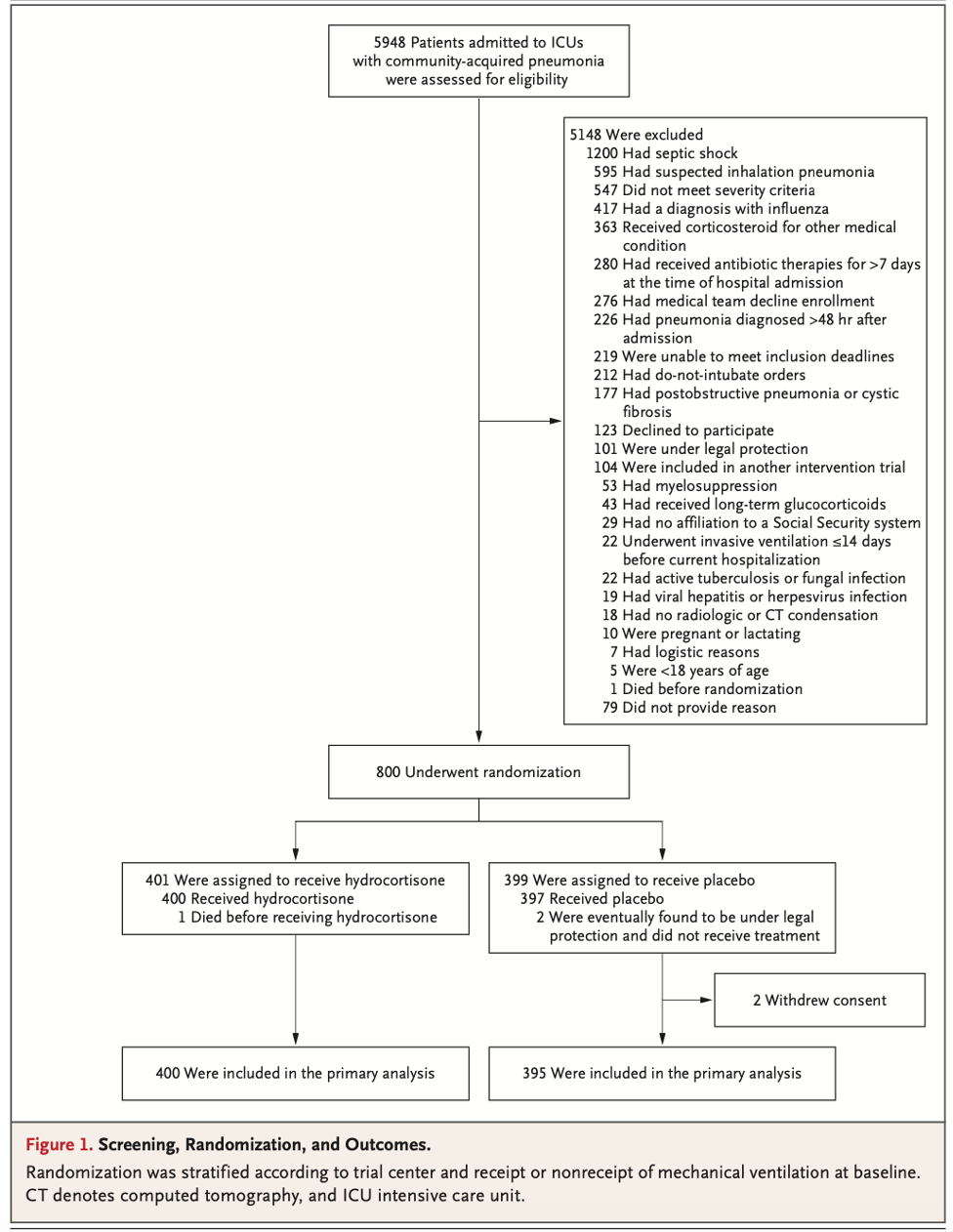
PICO:
Population:
- Enrolled patients in 3 French ICUs
- Inclusion criteria
- Age ≥ 18 years old
- Admitted to a participating ICU
- Diagnosed with severe community acquired pneumonia (CAP)
- The severity of pneumonia was defined by the presence of at least one of four criteria:
- Mechanical ventilation (invasive or non-invasive) with a positive end-expiratory pressure level of at least 5 cm of water
- The initiation of the administration of oxygen through a high-flow nasal with ARDS and a FiO2 of 50% or more
- If patients had a nonrebreathing mask the needed an estimated PaO2:FiO2 ratio of less than 300 (ARDS), according to prespecified charts
- A score of more than 130 on the Pulmonary Severity Index
- Diagnosis needed to be supported by radiographic criteria
- The severity of pneumonia was defined by the presence of at least one of four criteria:

- Exclusion criteria
- Do-not-intubate (DNI) status
- Pneumonia secondary to influenza
- Pneumonia secondary to aspiration
- Septic shock with vasoactive medication requirement on initial presentation
- Previously intubated within the prior 2 weeks (favoring ventilator associated PNA)
- Antibiotics for ≥ 1 week prior to presentation
- History of myelosuppression
- History of cystic fibrosis
- Active coinfection with:
- Tuberculosis
- Other fungal infection
- Viral hepatitis
- Herpes virus
- Pregnant/breastfeeding
- Patients needing anti-inflammatory corticosteroids or substitutive hydrocortisone another reason (think known Addison’s, adrenal suppression, chronic steroids)
- Patient on more than 15 mg/day of prednisone (or equivalent) for more than 30 days prior
- Hypersensitivity to corticosteroids
Randomization:
- Fairly well balanced
- Slightly higher rate of COPD in the intervention arm
- Slightly higher CRP in the intervention arm
- Overall similar PSIs, age, and comorbidities

Intervention:
- Standard self-described “state of the art” PNA care + respiratory support + hydrocortisone (200 mg/day for 4 days, followed by continuation for 4 or 8 days depending on response)
Comparator:
- Standard self-described “state of the art” PNA care + respiratory support + placebo
Outcome:
- Primary outcome of mortality at 28 days:
- 6.2% (95% CI 3.9-8.6) in the hydrocortisone group versus 11.9% (95% CI 8.7-15.1) in the placebo group
- NNT: 18
- Fragility index: 6
- Secondary outcomes:
- Less incidence of intubation and vasopressors in hydrocortisone group.

Take Aways:
- Positive trial
- This trial, while stopped early, showed a significant reduction in death at 28 days in patients admitted to the ICU for severe CAP.
- With a fragility index of 6, I find this data a little fragile to stop enrollment about 300 patients shy of projected enrollment. However, this is not a fault of the authors if this is what the safety board required. Still, it is possible the observed benefit [or magnitude of benefit] is due to chance given the early cessation.
- This is largely an examination of the effect of hydrocortisone in the ICU over several days. While I do see these patients on initial presentation, there is scant evidence directing the administration of hydrocortisone in the ED.
- This trial was conducted in France. Generally the patient population is more homogenous with about 85% of their population being white, compared to 59.3% non-Hispanic/Latino white in America [75.8% self-reported white alone].2,3 Perhaps the largest difference in the patient populations between the 2 countries is the rate of obesity with France having a 17% prevalence of obesity compared to 41.9% in the U.S.2,4 It is possible these differences in patient population can affect outcome.
- “State of the art care” was the described management of these patients with PNA, presumably this means they had access to all means of extracorporeal support. In many cases ECMO centers have improved outcomes for patients with ARDS [which all of these patients had by the defined enrollment criteria] which may have affected the outcomes.
My take:
- Based on these data, it is reasonable to give hydrocortisone to patients with severe PNA.
- While there is no way to know if a single dose of hydrocortisone would help these patients, inpatient medicine may be affected by the “good-idea” bias. Starting hydrocortisone in the ED may result in the continuation in the ICU, which in cases of these patients appears to be beneficial.
Source Article:
- Dequin PF, Meziani F, Quenot JP, et al. Hydrocortisone in Severe Community-Acquired Pneumonia [published online ahead of print, 2023 Mar 21].N Engl J Med. 2023;10.1056/NEJMoa2215145. doi:10.1056/NEJMoa2215145
- https://www.publichealth.columbia.edu/research/others/comparative-health-policy-library/france-summary
- https://www.census.gov/quickfacts/fact/table/US/RHI825221#RHI825221
- https://www.cdc.gov/obesity/data/adult.html








