Today on the emDOCs cast with Brit Long, MD (@long_brit), we discuss necrotizing soft tissue infection pearls and pitfalls with Jess Pelletier, MD.
Jess is an emergency medicine physician and Education Fellow at Washington University School of Medicine. This first part will look at some background, presentation, and laboratory evaluation for NSTI.
Episode 65: NSTI Pearls and Pitfalls Part I
Background:
- Necrotizing soft tissue infection is a collection of diseases.
- Incidence ranges between 0.024-0.045 per 1000 per year.
- Most patient are over age 50 years
- Variety of risk factors associated with NSTI
- Severe morbidity and mortality
- Rate of limb loss is 16%
- Mortality rate ranges between 20-100% without source control
Pearl #1: NSTI is a spectrum of diseases, of which necrotizing fasciitis is only one subtype.
Pitfall: Failure to understand the spectrum of pathology in NSTI.
- Type I: polymicrobial, most common, variety of risk factors (immunocompromised, pre-existing disease, older age)
- Type II: monomicrobial (Group A strep most common ,followed by MRSA), less common overall, risk factors (trauma, surgery, IV drug use)
- Type III: monomicrobial (Vibrio or Clostridium spp.), rare, contaminated water exposure, hemodynamic collapse before skin changes
- Type IV: Fungal (Candida or Zygomycetes spp.), very rare, immunocompromised or penetrating trauma
Pearl #2: Chronic illness or recent surgery should raise clinician suspicion for NSTI.
Pitfall: Discounting NSTI in the patient without a classic history.
- There may or may not be an inciting agent like a skin wound or trauma
- Transient bacteremia from GU or GI tract could lead to tissue seeding
- Among those diagnosed with NSTI, 80% have a clear point of inoculation such as a bite, surgical incision, injection site, or perianal source
- Variety of risk factors (diabetes, renal/liver disease, IV drug use, immunocompromise), but 25% have no known risk factor for NSTI
- Accounts for 1-2/10,000 inpatient admissions; 1/350 patients who present with back pain to an ED will have a spinal emergency.
- The mortality for SEA approximates 5%; 50% have residual deficits, with worse outcomes the longer the delay in diagnosis.

Pearl #3: There is wide variability in the clinical presentation of NSTI.
Pitfalls: Performing an incomplete skin examination and prematurely excluding the diagnosis in the absence of major skin findings.
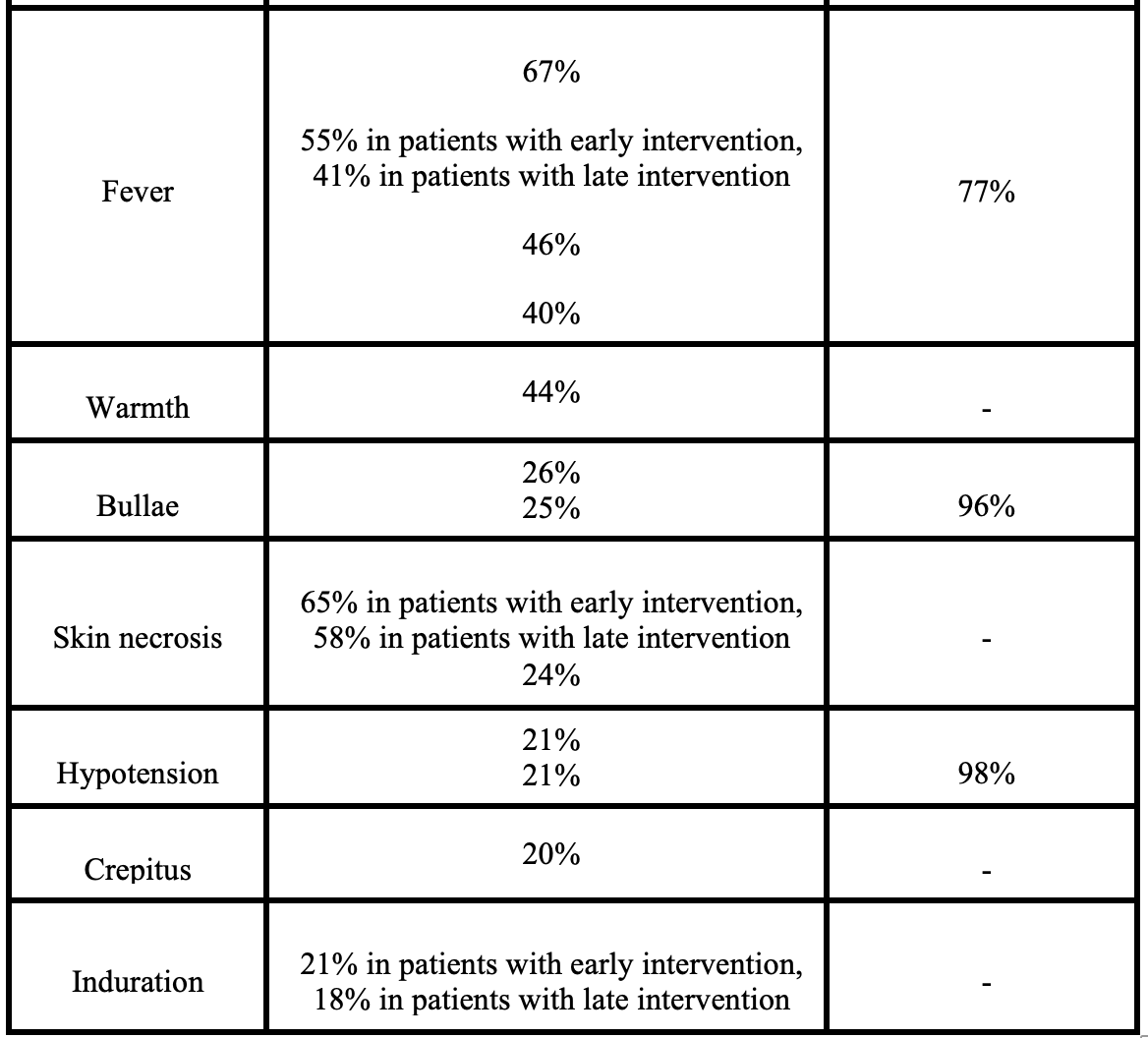
- NSTI is a clinical diagnosis, but many of the “classic” signs and symptoms are not present early in the disease course or will never occur.
- Nonspecific symptoms such as diarrhea, fatigue, loss of appetite, or malaise may precede the onset of skin findings in NSTI
- Pain is reported as the initial complaint in only 79% of cases
- 41-96% of NSTI cases are initially misdiagnosed as simple cellulitis or abscess
- Early skin findings may include mild erythema or edema
- Literature suggests swelling is the most common finding, followed by pain and erythema
- Warmth, bullae, skin necrosis, and crepitus are less common findings
- Fever has a specificity of 77% but sensitivity less than 50%
- Shock/hypotension occurs in less than 1/4 at time of presentation
- Toxic shock syndrome (with hypotension, macular rash, and palm and sole desquamation) is associated with up to half of GAS-associated NSTI cases
- Fournier’s gangrene may present differently than other subtypes; initial symptoms include perineal pruritus and pain, and skin changes are late
- Subacute NSTI can occur, with progression over weeks to months

Pearl #4: Laboratory testing cannot be used to rule out NSTI due to its low sensitivity.
Pitfall: Waiting for results of laboratory testing when NSTI is suspected.
- Diagnosis should be based on history and exam; don’t delay treatment for laboratory assessment
- There is no single laboratory test with adequate sensitivity and specificity to differentiate NSTI from other infectious processes or exclude the diagnosis
- Adjunctive laboratory testing can assist in determining severity of illness (end-organ damage) and shaping the pre-test probability of NSTI
- Obtain complete blood count, complete metabolic panel, c-reactive protein, lactate, and blood cultures
- Scoring systems are available, including the Laboratory Risk in Necrotizing Fasciitis (LRINEC) score
- Higher LRINEC scores tend to occur in sicker patients, but one meta-analysis found that the LRINEC score was only 68% sensitive and 85% specific for a score of 6 and 41% sensitive and 95% specific for a score of 8
- There have been case reports of patients with NSTI having LRINEC scores of 0
- LRINEC score can assist in communicating with the surgical specialist, but don’t use it to exclude the diagnosis

That’s it for Part I. Stay tuned for Part II, which looks at imaging and management.
References:
- Pelletier J, Gottlieb M, Long B, Perkins JC Jr. Necrotizing Soft Tissue Infections (NSTI): Pearls and Pitfalls for the Emergency Clinician. J Emerg Med. 2022 Apr;62(4):480-491.
- Fernando SM, Tran A, Cheng W, et al. Necrotizing Soft Tissue Infection: Diagnostic Accuracy of Physical Examination, Imaging, and LRINEC Score: A Systematic Review and Meta-Analysis. Ann Surg. 2019;269(1):58-65. doi:10.1097/SLA.0000000000002774
- Sin F, Yuen M, Lam K, Wu C, Tung W. A retrospective review of patients with necrotizing fasciitis presenting to an emergency department in Hong Kong. Hong Kong J Emerg Med. 2002;9(1):10-17.
- Mitchell A, Williams A, Dzendrowskyj P. Necrotising fasciitis: an 8.5-year retrospective case review in a New Zealand intensive care unit. Crit Care Resusc J Australas Acad Crit Care Med. 2011;13(4):232-237.
- Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014;101(1):e119-125. doi:10.1002/bjs.9371
- Misiakos EP, Bagias G, Patapis P, Sotiropoulos D, Kanavidis P, Machairas A. Current Concepts in the Management of Necrotizing Fasciitis. Front Surg. 2014;1. doi:10.3389/fsurg.2014.00036
- Peetermans M, de Prost N, Eckmann C, Norrby-Teglund A, Skrede S, De Waele JJ. Necrotizing skin and soft-tissue infections in the intensive care unit. Clin Microbiol Infect. 2020;26(1):8-17. doi:10.1016/j.cmi.2019.06.031
- Montravers P, Snauwaert A, Welsch C. Current guidelines and recommendations for the management of skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):131-138. doi:10.1097/QCO.0000000000000242
- Stevens DL, Bryant AE. Necrotizing Soft-Tissue Infections. N Engl J Med. 2018;378(10):971. doi:10.1056/NEJMc1800049
-
Chen K-CJ, Klingel M, McLeod S, Mindra S, Ng VK. Presentation and outcomes of necrotizing soft tissue infections. Int J Gen Med. 2017;10:215-220. doi:10.2147/IJGM.S131768
- Wilson MP, Schneir AB. A Case of Necrotizing Fasciitis with a LRINEC Score of Zero: Clinical Suspicion Should Trump Scoring Systems. J Emerg Med. 2013;44(5):928-931. doi:10.1016/j.jemermed.2012.09.039








