Authors: Erin R. Hanlin, MD (EM Attending Physician, San Antonio, TX) and Andrea Kaelin, MD (EM Attending Physician, Dayton, OH) // Edited by: Alex Koyfman, MD (@EMHighAK) and Brit Long, MD (@long_brit)
Case
A pleasant 84-year-old Caucasian female and her two daughters present to the emergency department for evaluation of intractable nausea and vomiting for the past week, now with one episode of hematemesis. Her daughters inform the provider of their mother’s recent diagnosis of lymphoma last month; she is just beginning to evaluate chemotherapy options with her oncologist. She appears clinically dehydrated and frail, and vital signs are notable for borderline fever at 100.0F. Mild RUQ and epigastric tenderness is elicited on physical exam. Her marked leukocytosis is not unexpected with WBC 24.5k. She exhibits mild hypokalemia at 3.1mg/dL, ketonuria, and a normal hepatic function panel with the exception of an elevated alkaline phosphatase of 352mg/dL. A lactic acid is elevated at 5.2 mg/dL.
Introduction
Portal vein thrombosis (PVT) refers to an occlusion at the trunk of the hepatic portal vein. It is a type of splanchnic vein thrombosis (SVT), which is a continuum of thrombotic diseases involving any combination of the portal, splenic, mesenteric, and suprahepatic veins.1 PVT can cause serious short and long-term morbidity in affected patients. Etiologies can be divided into three main categories: malignant thrombosis; non-malignant, non-cirrhotic thrombosis; and chronic cirrhotic liver disease.2 The clinical presentation and long term morbidity and mortality vary widely depending on the degree of extension into the other confluent splanchnic veins, the specific etiology of the vascular occlusion, and its chronicity; therefore, the diagnosis can be difficult to make in the Emergency Department (ED), and treatment decisions are not always clear.1-4
The overall population prevalence of PVT ranges from 1-1.7%; however, the occurrence of PVT is likely underestimated because the clinical presentation can vary widely and screening diagnostics have evolved and improved in recent years.5,6 The prevalence of PVT in non-malignant cirrhotic patients is as high as 26%. In patients with hepatocellular carcinoma and pancreatic adenocarcinoma, the prevalence is 14% and 11%, respectively.6,7
Anatomy and Epidemiology
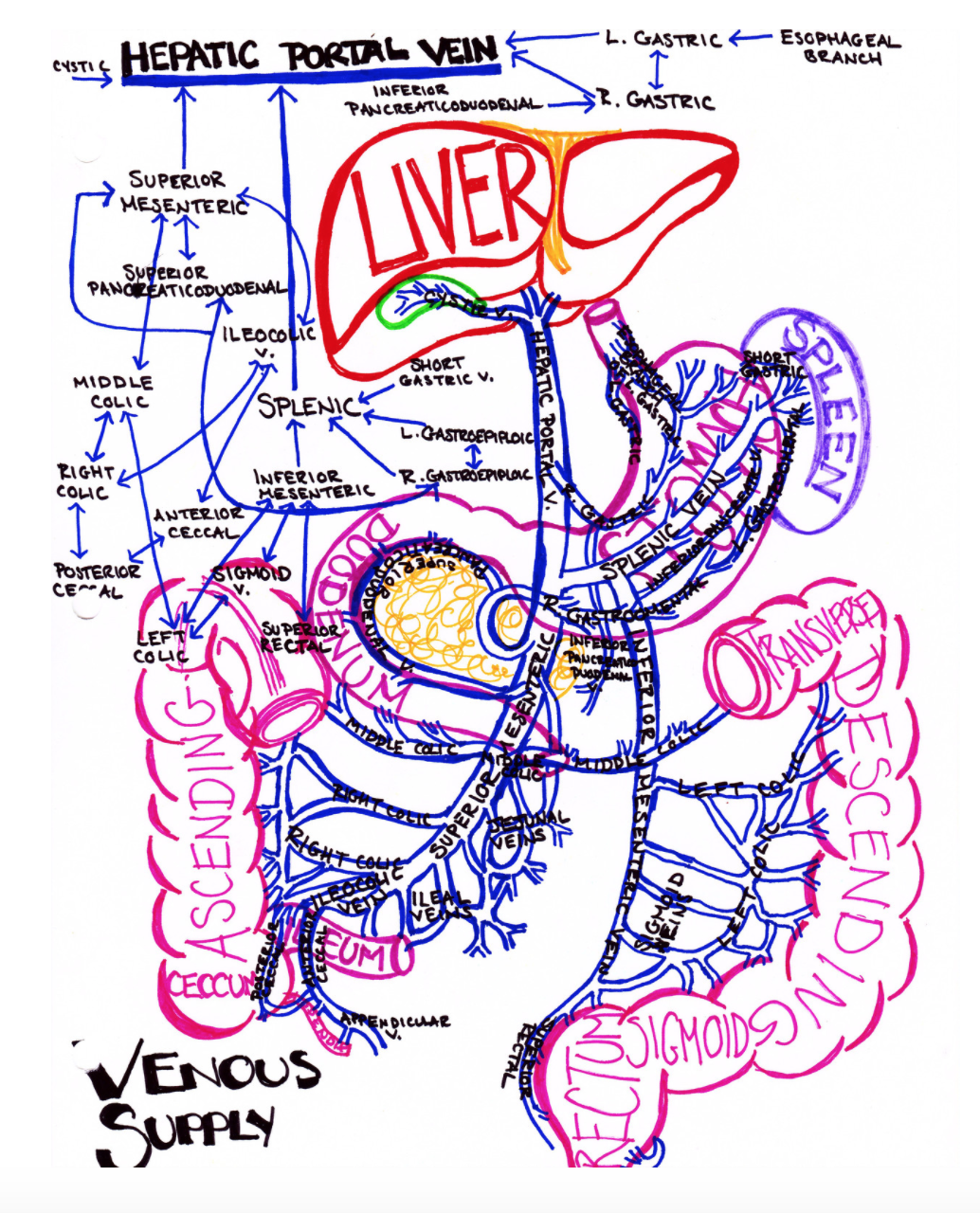
 The portal vein arises from the confluence of the superior mesenteric and splenic veins just posterior to the neck of the pancreas and accounts for nearly 75% of the blood supply to the liver.7 Although SVT is the overarching disease process that includes PVT, clinically PVT can refer to a thrombus in any location within the portal vein or its tributaries. Figure 1 demonstrates the prevalence of thrombosis in the various locations within the portal vein system among patients with PVT.6 Understanding this anatomy is important because the clinical presentation and long-term morbidity and mortality, and thus treatment, are greatly affected by the location of the thrombus.1-5
The portal vein arises from the confluence of the superior mesenteric and splenic veins just posterior to the neck of the pancreas and accounts for nearly 75% of the blood supply to the liver.7 Although SVT is the overarching disease process that includes PVT, clinically PVT can refer to a thrombus in any location within the portal vein or its tributaries. Figure 1 demonstrates the prevalence of thrombosis in the various locations within the portal vein system among patients with PVT.6 Understanding this anatomy is important because the clinical presentation and long-term morbidity and mortality, and thus treatment, are greatly affected by the location of the thrombus.1-5
The GI and Portal System, courtesy of Dr. Katelyn Hanson of Hanson’s Anatomy
Clinical Presentation
The clinical presentation of PVT varies greatly among patients depending on both etiology and specific venous segment involved. The table below (Figure 2) is taken from one large retrospective cohort study and demonstrates the wide variety of clinical signs and symptoms of PVT based on venous segment involved. Clinical presentation can range from asymptomatic to catastrophic GI bleed. In general, 20-40% of patients with PVT are asymptomatic, 20-50% present with abdominal pain, and 25-40% present with any form of GI bleed.1,8 Abdominal pain arises when the mesenteric vein is involved leading to venous congestion and eventually intestinal ischemia. Nearly half of patients with mesenteric vein involvement will have signs and symptoms of an acute abdomen.1 Splenomegaly is often present in both cirrhotic and non-cirrhotic PVT, whereas ascites is more likely seen in cirrhotic PVT.8 Complications secondary to portal hypertension are usually seen in the setting of extrahepatic portal and splenic vein thrombosis.2,3 This wide variety of clinical presentations makes PVT difficult to diagnose, and it is important to maintain a high index of suspicion in at risk populations.

Etiologies
Malignant PVT
PVT is a type of venous thromboembolic disease (VTE), and thus any underlying pro-thrombotic condition such as malignancy is a risk factor. In one study of 832 patients with splanchnic vein thrombosis, cancer was the most common etiology accounting for 27% of cases. Of these patients, the three most common sites of malignancy were pancreatic, hepatobiliary, and intestinal, and the development of PVT was associated with the progression of disease.1 PVT is common in hepatocellular carcinoma and is an extremely poor prognostic indicator.8 Complications from portal hypertension are low in cancer patients with PVT without concomitant cirrhosis. For example, in one large autopsy study, upper GI bleeds occurred in only 14% of non-cirrhotic cancer patients with PVT, as compared to 59% of cirrhotic cancer patients with PVT.2
In addition to local malignancies, myeloproliferative disorders and other hematologic malignancies are also prominent causes of hepatic vein thrombosis and often result in multifocal thrombosis.1,7,8 It is estimated that up to 3% of patients with PVT have an underlying hematologic malignancy; therefore, in this patient population presenting with abdominal pain, new ascites, or GI bleed, PVT should be on the differential diagnosis.3,8
Cirrhotic PVT
PVT is an important complication of liver cirrhosis. It is seen in up to 5% of compensated cirrhotic patients and in over 25% of patients with advanced disease.9,10 In patients with cirrhosis secondary to hepatocellular carcinoma, the incidence was as high as 36%.3,11,12 The increased incidence seen in advanced liver disease is likely due to the combination of slow blood flow through fibrotic hepatic venules, leading to vascular endothelial damage and unbalanced pro- and anticoagulative factors.11,13 PVT may be completely asymptomatic in patients with liver cirrhosis; however, in more than half of cases patients present with serious complications such as gastrointestinal hemorrhage and intestinal infarction. Mesenteric vein involvement leads to intestinal infarction and is rarely asymptomatic in this population.14
One subset of cirrhotic patients in which PVT causes a particularly large disease burden is in the liver transplant population. Up to 70% of explanted livers are found to have PVT upon subsequent pathology examination.13,15 PVT is a recognized complication in cirrhotic patients awaiting liver transplantation. This is an important diagnosis within this population because the extent of the thrombosis can negatively affect patients’ eligibility for liver transplantation. Treatment is aimed at recanalization to reestablish at least partial blood flow for future grafting.8
Non-Cirrhotic Non-Malignant PVT
Causes of non-cirrhotic non-malignant PVT include various inflammatory and surgical conditions such as diverticulitis, appendicitis, pancreatitis, Crohn’s disease, splenectomy, liver transplant, and cholecystectomy. General prothrombotic conditions including factor V Leiden, systemic contraceptive use, protein C and S disease, and recent pregnancy are also important underlying etiologies of PVT.1,2,8 Spontaneous PVT without concomitant liver disease usually results in mild disease with minimal to no liver dysfunction.9,16 Left untreated, collateral vessels will form a hepatic cavernoma; however, sufficient blood flow is usually maintained.9 As in the setting of other spontaneous VTE, the diagnosis of PVT without a clear inciting factor should prompt a hypercoagulable workup.
Diagnosis
The American Association for the Study of Liver Disease (AASLD) recommends clinical consideration of acute PVT in “any patient with abdominal pain of more than 24 hours duration,” regardless of concomitant fever or ileus.17 Unfortunately, physical examination traits of patients presenting with PVT are highly variable as is the symptomatology; these are dependent on the timing of onset and extension of occlusion. Similarly, laboratory analysis exhibits a spectrum of mostly non-patterned and non-specific values of complete blood count and comprehensive metabolic panel with normal transaminase levels, unless an underlying condition such as cirrhotic liver failure or hepatitis is present.18 Liver function is usually preserved in PVT likely due to collateral circulation that forms within days of acute occlusion and the preserved arterial blood flow.19 Abnormalities in the coagulation studies may be present in accordance with underlying thrombophilic disorders and an elevated D-dimer can also trigger additional consideration for thrombotic causes of abdominal pain. Febrile variants of clinical presentation should also have septic thrombi considered and be evaluated appropriately with routine blood cultures.17
The AASLD recommends evaluating for PVT with non-contrast and contrast enhanced CT of the abdomen, although they consider doppler ultrasonography (US) an acceptable alternative.17 Color doppler US may demonstrate the hyperechoic thrombus in a dilated portal vein with or without collateral circulation as well as partial or absent flow within the vessel.18 The reported sensitivity ranges between 60-100%.16 Portal flow velocity below 15 cm/s on doppler US was identified by Zocco et al. to discern presence of venous stasis as a contributing factor for formation of PVT in the evaluation of a cirrhotic patient population.20
In patients with more severe symptoms, those who look clinically concerning, or those who have clinical or laboratory evidence of multisystem organ failure or shock should be evaluated with CT. Mesenteric ischemia and bowel perforation sequelae of PVT may be detectable on CT imaging as opposed to US, and these patients require emergent surgical consultation.18 As in other patients with upper GI bleeding, patients with concomitant PVT should be considered for endoscopic evaluation and potentially banding of varices.
For evaluation of the thrombus extension into the mesenteric circulation in stable patients, however, contrast enhanced magnetic resonance (MR) imaging is recommended.17 This further characterization of clot burden is critical to evaluate and define interventional and treatment options. Endoscopic US as compared to regular color doppler US may also be used with improved sensitivity of 81% and specificity of 93% and can assist in the visualization of non-occluding thrombi and local tumor invasion; however, evaluation of the superior mesenteric vein and intrahepatic portal veins is still limited.21 Both MR and specialized endoscopic US are important adjuncts in the diagnosis and evaluation of PVT; however, these specialized modalities need not be performed in the ED.
Chronic PVT may present asymptomatically and as an incidental radiographic finding in cirrhotic patients and should be considered in portal hypertension patients as well.11,17 Color doppler US and contrast CT can both demonstrate the collateral circulation which forms a hepatic cavernoma of tortuous adjacent vessels with the absence of normal portal vein anatomy. MR angiography may further be used beyond the ED to evaluate patency of prior surgical shunts and flow direction.22
Treatment
In general, treatment is aimed at recanalization and prevention of thrombotic extension, especially into the mesenteric vein. There is conflicting data on the risks and benefits of anticoagulation, but general consensus is to begin anticoagulation as early as possible regardless of which venous segment is involved.1,4,5,13,23 One retrospective case controlled study investigated survival and recurrence in patients with first time SVT and found that patients receiving warfarin for SVT had a significantly higher overall survival rate than patients who were not anticoagulated.1 Early anticoagulation in patients with mesenteric vein thrombosis is particularly important as it has been shown to decrease complications of bowel necrosis and subsequent peritonitis.24 Interestingly, surgery alone has not been shown to impact mortality in mesenteric vein thrombosis complicated by bowel necrosis; however, when combined with early pre-operative anticoagulation, surgical intervention demonstrates a consistent survival benefit.1,25
Unfractionated heparin or low molecular weight heparin (LMWH) bridge to warfarin is the generally accepted approach to anticoagulation for acute PVT. Doses are the same as for other VTE diseases (unfractionated heparin 80 units/kg IV to max dose of 5000 units followed by continuous infusion of 18 units/kg/hr; LMWH 1mg/kg SC q12hr). LMWH is equally efficacious and is preferred over unfractionated heparin due to ease of administration and lower incidence of heparin-induced thrombocytopenia.9 Once warfarin is started, the target INR is between 2-3.1,9 Anticoagulation is usually continued for 6 months or longer if there is recurrence or disease progression.9
In cirrhotic patients without concomitant portal hypertension, anticoagulation is recommended as soon as possible in the setting of acute PVT. It is estimated that the rate of recanalization can decrease from around 70% to 25% if anticoagulation is delayed by as little as a week.8,25,26 Although it is not necessary to start anticoagulation immediately in the ED, these patients will require admission in order to begin anticoagulation as soon as possible.
There is less consensus on the use of anticoagulation in the setting of chronic PVT. As chronic PVT is often associated with pre-existing portal hypertension and its complications, the risk of GI bleed often outweighs the benefits of anticoagulation. Some experts advocated for endoscopic evaluation and eradication of varices prior to initiation of anticoagulant therapy.25 In the ED, these patients should not be started on new anticoagulant therapy without close consultation with the patient’s specialist.
Other treatment modalities are available including thrombectomy, local or systemic thrombolysis, balloon angioplasty, or stent placement.9 Cirrhotic patients also may benefit from transjugular intrahepatic portosystemic shunt (TIPS), which not only treats portal hypertension, thus preventing its subsequent sequelae, but also promotes venous recanalization. Although direct-acting oral anticoagulants (DOAC’s) are beginning to be used for the treatment of PVT, there has been no large study investigating their efficacy and safety in this specific VTE disease.26 With their widespread use in other thrombotic diseases and the recent introduction of a reversal agent, DOAC’s will likely gain popularity in the near future.
Patients with new acute PVT will require admission most commonly to a surgical service; however, it is acceptable to admit to a medicine service with a surgery consult. Although the mainstay of therapy for non-critical patents with new PVT is medical management with anticoagulants, these patients are at high risk of thrombotic extension into the mesenteric vein and subsequent bowel ischemia; therefore, they will require frequent abdominal checks and evaluation by a surgeon.
Case Conclusion
In the setting of newly diagnosed lymphoma and abdominal pain with mild focal symptoms in the RUQ, our patient underwent comprehensive liver US and was found to have a new thrombus in the portal vein. She was started on heparin in the emergency department and admitted to her oncologist’s service with a surgery and hepatology consult made by the inpatient team. She underwent further MR angiography as an inpatient and was found to have only minimal clot extension into the mesenteric vein. Endoscopic evaluation was also conducted in the setting of her episode of hematemesis, revealing mild portal hypertensive gastropathy. No varices were found. She remained stable and her abdominal pain and vomiting improved during her clinical course. She was discharged to home on warfarin with close follow up with her oncologist and hepatologist.
Clinical Pearls
– Patients with PVT are often asymptomatic. Knowledge of risk factors associated with the disease is important to ensure the disease is considered.
– Abdominal pain in patients with PVT is often associated with mesenteric vein occlusion and subsequent intestinal ischemia.
– Consider PVT in patients with hematologic cancers or other pro-thrombotic conditions presenting to the ED with abdominal pain or new ascites.
– Color Dopper US is sufficient for diagnosing PVT in clinically stable patients.
– Consider contrast-enhanced CT in patients with severe symptoms or those who are clinically deteriorating to look for sequelae of PVT.
– LFTs are usually normal in patients with PVT.
– Timely initiation of anticoagulation with heparin or LMWH followed by warfarin is the recommended treatment for acute PVT.
– Patients with acute PVT should be admitted to surgery or a medicine service with a surgery consult for frequent abdominal checks and initiation of anticoagulation therapy.
References / Further Reading
- Thatipelli, Mallikarjun R., Robert D. McBane, David O. Hodge, and Waldemar E. Wysokinski. “Survival and Recurrence in Patients With Splanchnic Vein Thromboses.” Clin Gastroenterol Hepatol 2 (2010): 200–205.
- Trebicka, Jonel, and Christian P. Strassburg. “Etiology and Complications of Portal Vein Thrombosis.” Viszeralmedizin6 (2014): 375–380. PMC. Web. 19 Aug. 2017.
- Pieri, Giulia, Eleni Theocharidou and Andrew K. Burroughs. “Liver in Haematological Disorders.” Best Pract Res Clin Gastroenterol 4 (2013): 513-3.
- Riva, Nicoletta, Marco P. Donadini, Francesco Dentali, Alessandro Squizzato, Walter Ageno. “Clinical Approach to Splanchnic Vein Thrombosis: Risk Factors and Treatment.” Thromb Res.1 (2012): S1-3.
- Plessier, Aurélie, Pierre-Emmanuel Rautou, Dominique-Charles Valla. “Management of Hepatic Vascular Diseases.” J Hepatol1 (2012): S25–S38.
- Ageno, Walter, Alessandro Squizzato, A. Togna, F. Magistrali, M. Mangini, C. Fugazzola, F. Dentali. “Incidental Diagnosis of a Deep Vein Thrombosis in Consecutive Patients Undergoing a Computed Tomography Scan of the Abdomen: A Retrospective Cohort Study.” J Thromb Haemost. 10 (2012): 158–160.
- Ögren, Mats, David Bergqvist, Martin Björck, Stefan Acosta, Henry Eriksson, Nils H Sternby. “Portal Vein Thrombosis: Prevalence, Patient Characteristics and Lifetime Risk: A Population Study Based on 23,796 Consecutive Autopsies.” World J Gastroenterol. 12 (2006): 2115–2119.
- Manzano-Robleda, M del C., Beatriz Barranco-Fragoso, Misael Uribe, Nahum Méndez-Sánchez. “Portal Vein Thrombosis: What Is New.” Ann Hepatol. 1 (2015): 20-7.
- Senzolo, Marco, Oliviero Riggio1, Massimo Primignani. “Vascular Disorders of the Liver: Recommendations from the Italian Association for the Study of the Liver (AISF) Ad Hoc Committee.” Dig Liver Dis.7 (2011): 503–514.
- Garcia-Pagan, Juan Carlos., Manuel Hernandez-Guerra., and Jaime Bosch,. ”Extrahepatic Portal Vein Thrombosis.” Semin Liver Dis. 28 (2008): 282–292.
- Tsochatzis, Emanuel A., Marco Senzolo, Giacomo Germani, Alexander Gatt, Andrew K. Burroughs. “Systematic Review: Portal Vein Thrombosis in Cirrhosis.” Aliment Pharmacol Ther. 31 (2010): 366–374.
- Francoz, Claire, Dominique Valla, Francois Durand. “Portal Vein Thrombosis, Cirrhosis and Liver Transplantation.” J Hepatol. 57 (2012): 203–212.
- Francoz, Claire, Jacques Belghiti, Valerie Vilgrain, Daniel Sommacale, Valerie Paradis, Bertrand Condat, H Denninger, Alain Sauvanet, Dominique Valla and Francois Durand. “Splanchnic Vein Thrombosis in Candidates for Liver Transplantation: Usefulness of Screening and Anticoagulation.” Gut. 54 (2005): 691–697.
- Amitrano, Lucio, Maria Anna Guardascione, Vincenzo Brancaccio, Maurizio Margaglione, Francesco Manguso, Luigi Iannaccone, Elvira Grandone and Antonio Balzano. “Risk Factors and Clinical Presentation of Portal Vein Thrombosis in Patients with Liver Cirrhosis.” J Hepatol5 (2004): 736 – 741.
- Primignani, Massimo. “Portal Vein Thrombosis, Revisited.” Dig Liver Dis. 42 (2010): 163–170.
- Chawla, Yogesh., Ajay Duseja, Radha K. Dhiman. “Review Article: The Modern Management of Portal Vein Thrombosis.” Aliment Pharmacol Ther. (2009): 881-894.
- DeLeve, Laurie D., Dominique Valla, Guadalupe Garcia-Tsao. “Vascular Disorders of the Liver” 49 (2009):1729-1764.
- Kocher, Gregor, Andreas Himmelmann. “Portal Vein Thrombosis (PVT); a study of 20 non-cirrhotic cases. Swiss Med Wkly. 135 (2005): 372-376.
- Kumar, Shaji, Michael G. Sarr, Patrick S. Kamath. “Mesenteric Venous Thrombosis.” N Engl J Med. 345 (2001): 1683-1688.
- Zocco, Maria A., Enrico Di Stasio, Raimondo De Cristofaro, Marialuisa Novi, Maria Elena Alnora, Francesca R. Ponziani, Laura Riccardi, et al. “Thrombotic Risk Factors in Patients with Liver Cirrhosis: Correlation with MELD Scoring System and Portal Vein Thrombosis Development.” Hepatol. (2009) 51:682-9.
- Lai, Lawrence, William R Brugge. “Endoscopic Ultrasound is a Sensitive and Specific Test to Diagnose Portal Venous System Thrombosis (PVST) Am J Gastroenterol. 99 (2004): 40-44.
- Haddad, Maurice C., David C. Clark, Hassan S. Sharif, Mona al Shahed, Osarugue Aideyan, Bassam M. Sammak. “MR, CT, and Ultrasonography of Splanchnic Venous Thrombosis” Gastrointest Radiol 17 (1992): 34-40.
- Rangari, Manisha, Ruchika Gupta, Monika Jain, Veena Malhotra, and Shiv K. Sarin. “Hepatic Dysfunction in Patients with Extrahepatic Portal Venous Obstruction.” Liver Int. 23 (2003): 434–439.
- Qi, Xingshun, Guohong Han, Daiming Fan. “Management of Portal Vein Thrombosis in Liver Cirrhosis.” Nat Rev Gastroenterol Hepatol. 7 (2014): 435-46.
- Ponziani, Francesca R., Maria A Zocco, Chiara Campanale, Emanuele Rinninella, Annalisa Tortora, Luca Di Maurizio, Giuseppe Bombardieri, Raimondo De Cristofaro, Anna M De Gaetano, Raffaele Landolfi, and Antonio Gasbarrini. “Portal Vein Thrombosis: Insight into Physiopathology, Diagnosis and Treatment.” World J Gastroenterol. 2 (2010): 143–155.
- Nery, Filipe, Diana Valadares, Sara Morais, Manuel Teixeira Gomes, Andrea De Gottardi. “Efficacy and Safety of Direct-Acting Oral Anticoagulants Use in Acute Portal Vein Thrombosis Unrelated to Cirrhosis.” Gastroenterology Res.2 (2017): 141-143.









2 thoughts on “Portal Vein Thrombosis: A Primer for Emergency Medicine Physicians”
Pingback: Portavenstrombos – Mind palace of an ER doc
EXCELENTE REVISION