Author: Alexander M. Mozeika, PharmD, MS4 Rutgers New Jersey Medical School // Editor: Cynthia Santos, MD (@CynthiaSantosMD, Assistant Professor, Emergency Medicine, Medical Toxicology, Rutgers NJMS); Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)

Case:
A 23-year-old man who has been on a strict diet for several months presents with vertical nystagmus, tremors, and diplopia. He has a history of epilepsy managed with phenytoin. He reports compliance with his medications and takes them as prescribed. His phenytoin levels for the last five years have always ranged between 16-18 mg/dL.
Questions:
- What are the pharmacokinetic factors affecting distribution?
- How are these factors altered in the setting of poisoning (i.e. toxicokinetics)?
Background:1-3
- Distribution describes the transfer of a xenobiotic in body sites
- Single or multi-compartment models can mathematically describe the distribution
- Xenobiotics reach steady state after 3-5 half-lives (~5 in most cases)
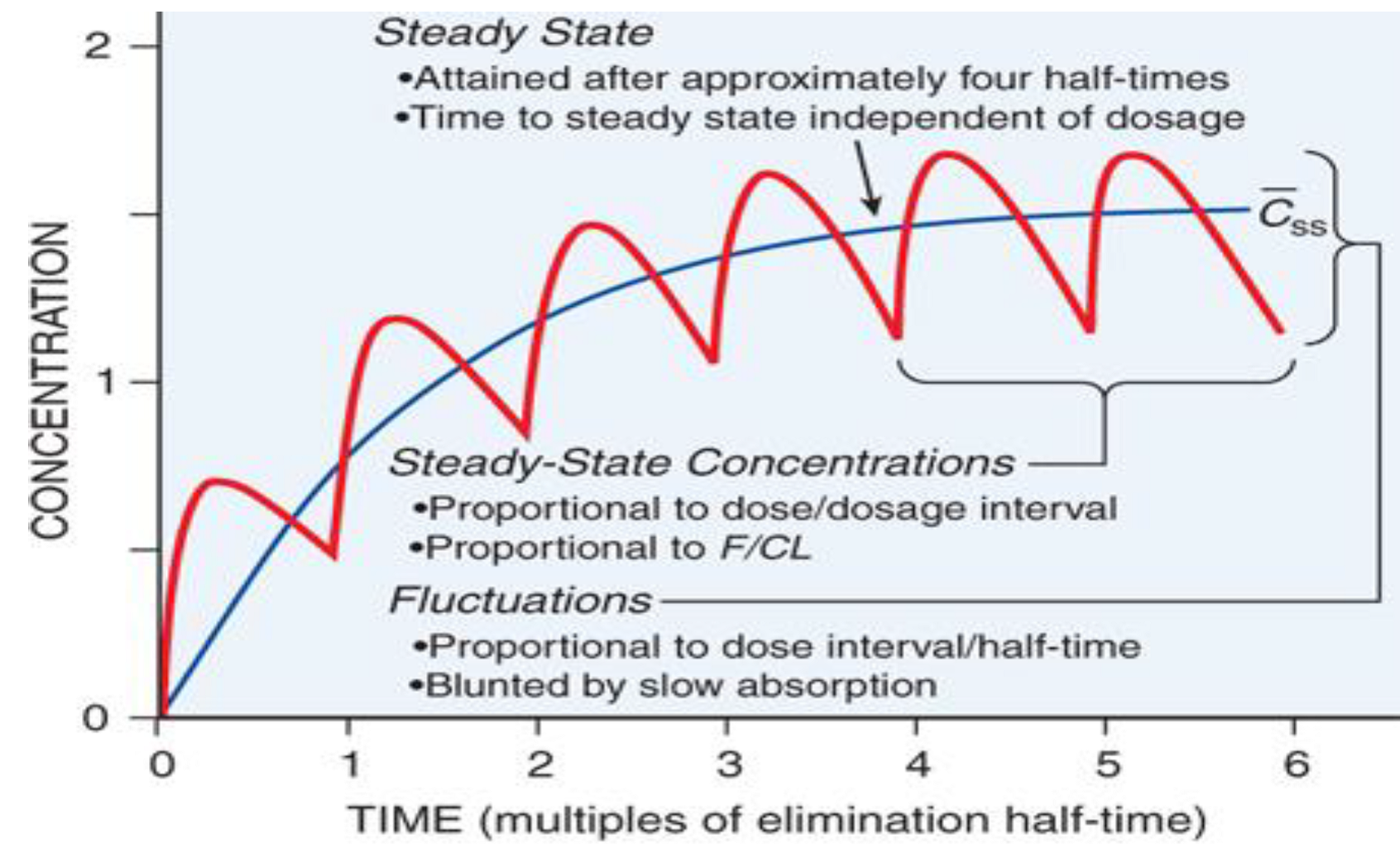
- Various factors affect distribution including protein-binding, lipophilicity, etc.
- Volume of distribution (Vd) – a conceptual descriptor of where the substance is in the body relative to the plasma
- It is an “apparent” or theoretical volume and can be larger than the entire body
Toxicokinetics of Distribution:
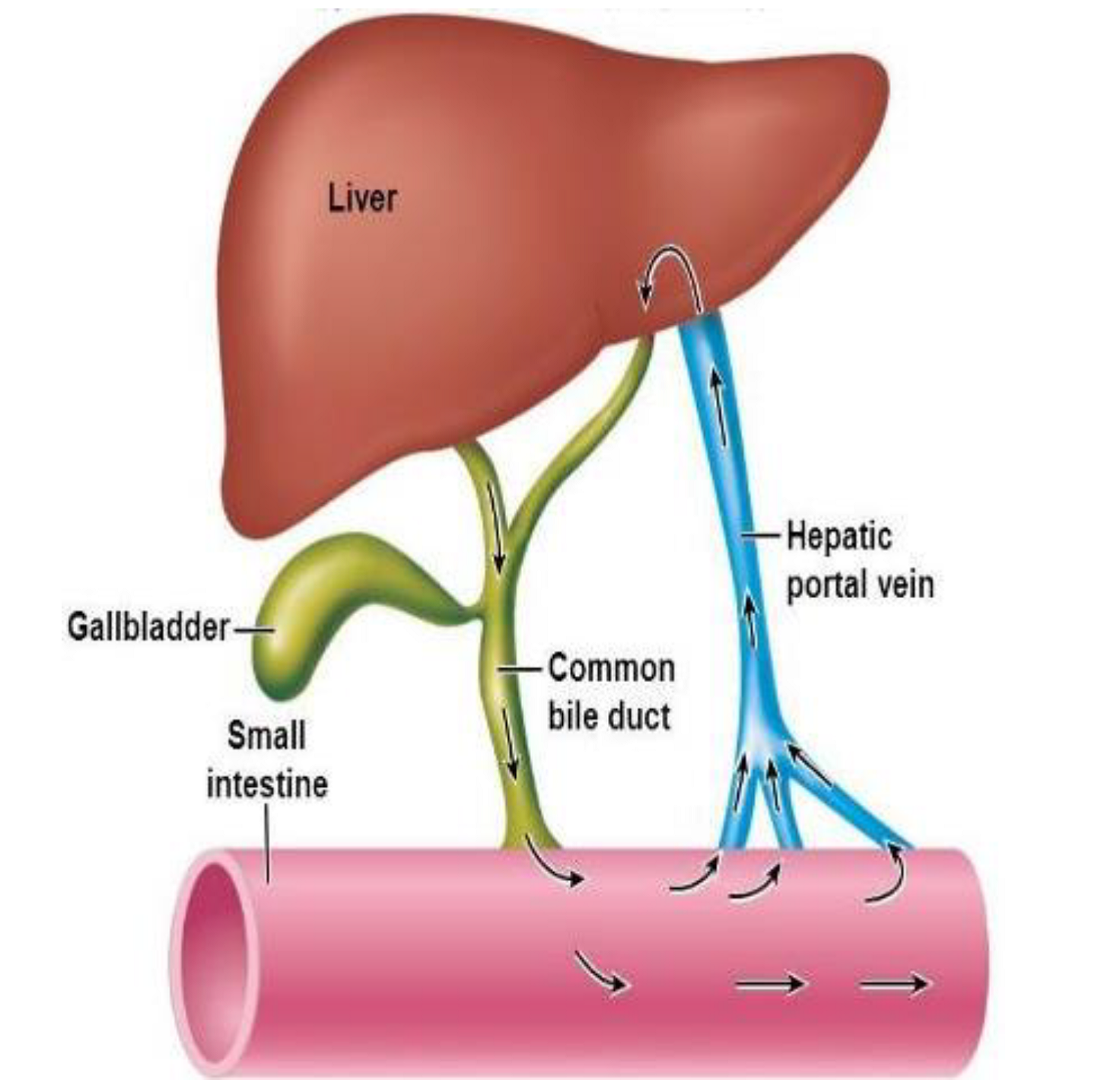
- Enterohepatic Recirculation:4
- Involves substances (e.g. bile salts, drugs) that are metabolized by the liver, excreted into the bile, and delivered to the intestines; there they are reabsorbed across the intestinal mucosa and returned to the liver via portal circulation (see figure below)
- Numerous xenobiotics undergo enterohepatic recirculation
- Ex: valproic acid, warfarin, digoxin, amiodarone, morphine, rifampin
- These medications may exhibit prolonged toxicity
- Given their reentry into the GI tract, multi-dose activated charcoal is a potential therapy for substances that have high enterohepatic recirculation
- Activated charcoal dose : 12.5 g/hr in divided doses (e.g. 50g Q4hr)
- Volume of Distribution:1
- Pearl: Volume of distribution is a large factor determining the feasibility of a substance being dialyzed
-
- Substances with large Vd (>1 L/kg) have lower concentrations in the plasma and thus are unlikely to be dialyzable
- Substances with small Vd are more likely to be dialyzed because they primarily exist in the plasma space
- Ex: alcohol, lithium, salicylates, phenytoin, phenobarbital, and valproic acid
- Protein-binding:1
- Substances can bind to plasma proteins, namely albumin
-
-
- Thus, changes in albumin can affect overall free xenobiotic concentrations
- Also, substances that compete for the same protein-binding sites can greatly increase each other’s free concentrations
- Following exposure to a high-dose of xenobiotic, binding sites can be saturated leading to non-linear kinetics
-
Case (continued):5
The patient has been severely malnourished, and follow-up labs revealed an albumin of 2 g/dL. Hypoalbuminemia causes alterations in protein-binding of phenytoin. An albumin of 2 g/dL results in approximately a doubling of free phenytoin.
- Corrected phenytoin = measured phenytoin/[(0.2 x albumin) + 0.1]
- At albumin of 4.0 g/dL with measured phenytoin of 16 mg/dL, the corrected phenytoin level is 17.8 mg/dL
- At albumin of 2.0 g/dL with measured phenytoin of 16 mg/dL, the corrected phenytoin level is 32 mg/dL
Main Point:
- Distribution is an abstract pharmacokinetic principle that has numerous implications in toxicological emergencies – both in toxicity course and management
- Substances with small volumes of distribution and small molecular weights may easily be dialyzed
- Substances that undergo enterohepatic recirculation may be eliminated through multi-dose activated charcoal with an extended window of administration
- Protein-binding is an important pathoetiology of toxicity secondary to high-dose ingestions and drug-drug interactions
References:
- Howland MA. Pharmacokinetic and Toxicokinetic Principles. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies, 10e. New York, NY: McGraw-Hill Education; 2015.
- Shargel L, Yu ABC. Introduction to Biopharmaceutics and Pharmacokinetics. In: Shargel L, Yu ABC, eds. Applied Biopharmaceutics & Pharmacokinetics, 7e. New York, NY: McGraw-Hill Education; 2016.
- Hilal-Dandan R, Brunton LL. Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, Metabolism, and Elimination. Goodman and Gilman’s Manual of Pharmacology and Therapeutics, 2e. New York, NY: McGraw-Hill Education; 2016.
- Gao Y, Shao J, Jiang Z, et al. Drug enterohepatic circulation and disposition: constituents of systems pharmacokinetics. Drug discovery today. 2014;19(3):326-340.
- Kiang TK, Ensom MH. A comprehensive review on the predictive performance of the Sheiner-Tozer and derivative equations for the correction of phenytoin concentrations. Annals of Pharmacotherapy. 2016;50(4):311-325.









2 thoughts on “TOXCard: Distribution Toxicokinetics”
I think you used the wrong equation for albumin and phenytoin. You wrote 0.2xalbumin. I think it is 0.25
Thanks for pointing it out, we used the equation for patients with uremia or significant ESRD (the patient was severely malnourished). The factor is different for normal patients.
Patients without end-stage renal disease:
Corrected phenytoin (equation1)= [ (Measured phenytoin) / (0.25∗Albumin+0.1)]
Corrected phenytoin (equation2)= [ (Measured phenytoin) / (0.29∗Albumin+0.1)]
Patients WITH end-stage renal disease or significant uremia:
Corrected phenytoin= [ (Measured phenytoin)/ (0.2∗Albumin+0.1)]
https://clincalc.com/Phenytoin/Correction.aspx