Authors: Matthew Cravens, MD (Emergency Medicine Resident, Atrium Health’s Carolinas Medical Center); Christine Murphy, MD (Emergency Medicine Attending, Medical Toxicologist, Atrium Health’s Carolinas Medical Center) // Reviewed by: James Dazhe Cao, MD (@JamesCaoMD, Associate Professor of EM, Medical Toxicology, UT Southwestern Medical Center, Dallas, TX); Alex Koyfman, MD (@EMHighAK); and Brit Long, MD (@long_brit)

Case:
A 78-year-old man presented to the emergency department after ingesting six boxes of old rat bait he found in his garage (active ingredient: 0.005% brodifacoum) over the past few days in a suicide attempt. He had no active bleeding. Initial vital signs were: BP 96/43, HR 98, T 97.4°F, RR 16.
Questions:
- What are superwarfarins, and how do they cause toxicity?
- What labs should be ordered for this patient?
- Should the patient be given empiric vitamin K prior to lab result?
Background:
- Developed in the 1950s and 1960s, anticoagulant rodenticides have been used for years to help control rodent infestations.
- Anticoagulant rodenticides cause death by internal bleeding and inhibited coagulation several days after ingestion.
- There are two generations of anticoagulant rodenticides:
- First generation:
- Hydroxycoumarins (e.g., warfarin)
- Indandiones (e.g., diphacinone, chlorophacinone)
- Second generation: superwarfarins or long-acting vitamin K antagonists (e.g., brodifacoum, bromadiolone)1
- First generation:
- Superwarfarins were developed as more potent and longer-acting rodenticides in the 1970s after rodents evolved warfarin resistance.2
- In mice given one-half the lethal dose 50 (LD50), the plasma elimination half-life of brodifacoum was 92 days compared to 15 days for warfarin.3 Of note, warfarin’s elimination half-life was longer than expected in this study due to a bi-phasic decline – warfarin reached very low concentrations within 5 days of administration, then entered a second, much slower elimination phase.3
- In humans, the elimination half-life of brodifacoum has been estimated between 16 and 62 days,4 compared to 35 hours for warfarin.5
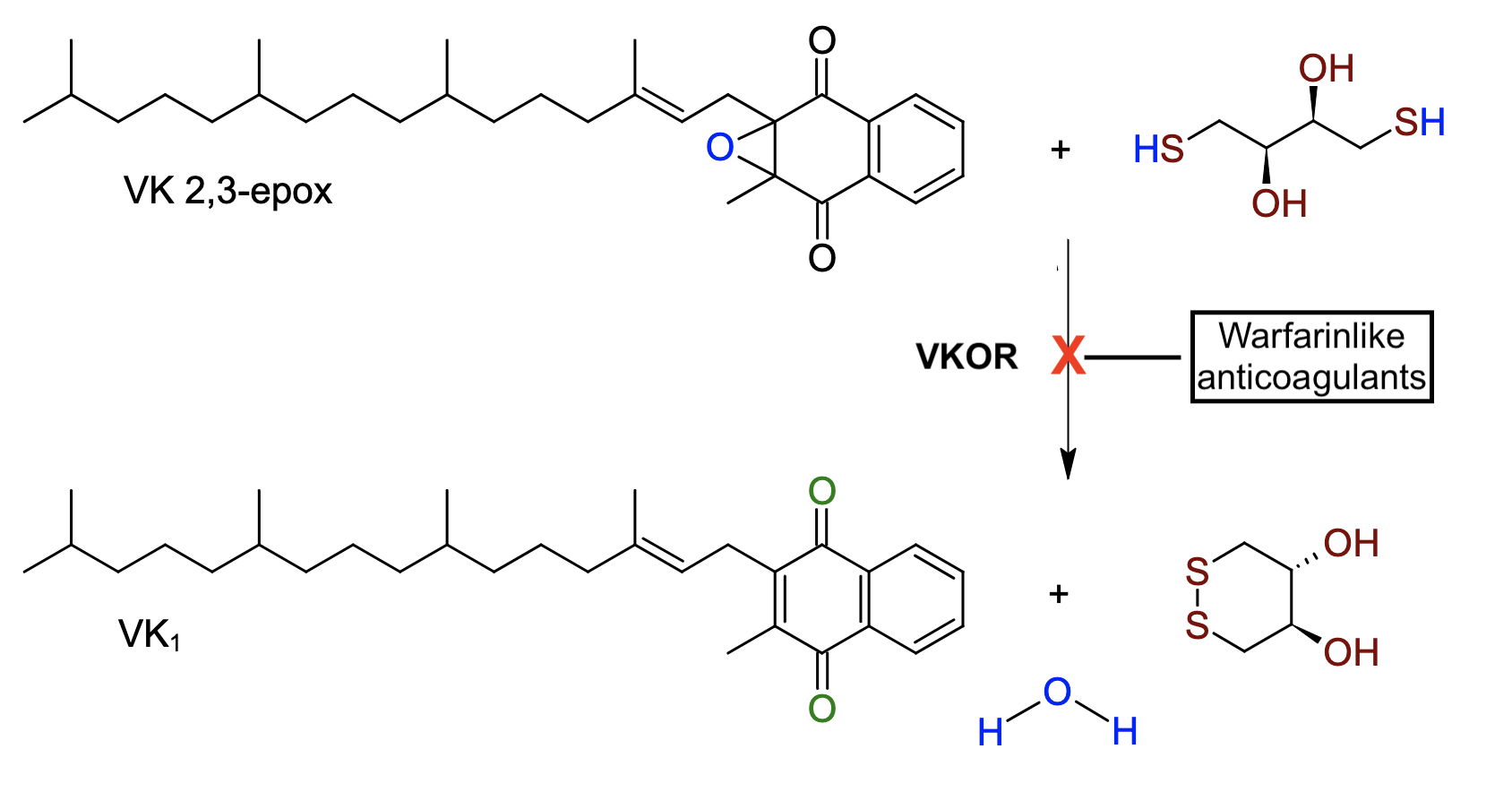
- Superwarfarins are vitamin K epoxide reductase (VKOR) inhibitors with the same mechanism of action as warfarin (Figure 1). They cause anticoagulation by preventing the recycling of vitamin K, depressing the hepatic activation of factors II, VII, IX, and X.1
- While the U.S. Environmental Protection Agency (EPA) banned the sale of superwarfarins from use in residential consumer products in 2011:6
- Warfarin and indandione rodenticides are still sold in the U.S.
- The threat of superwarfarin exposure from pre-2011 stored rat bait persists.
- In the early 2000s, prior to the EPA ban, about human superwarfarin exposures occurred annually in the U.S. Most unintentional exposures are pediatric, and of these, less than 1% have a moderate or major effect.7
- Superwarfarins may be ingested, inhaled, or absorbed topically, and possess bioterrorism potential.8
- In 2018, inhalation of superwarfarin-contaminated synthetic cannabinoids (“spice”) affected 300 patients and caused 7 deaths in an outbreak centered in Illinois, U.S.8
Clinical Presentation and Diagnosis:
- Symptoms of easy bruising or bleeding do not develop for several days after exposure.
- Patients naïve to vitamin K antagonists presenting on the day of exposure, like a child with a witnessed ingestion, are asymptomatic and have normal INRs.
- Patients presenting after an intentional ingestion can have major bleeding
- Most commonly a genitourinary source, with flank or abdominal pain and hematuria.
- Gastrointestinal and intracranial hemorrhage are also seen.
- No confirmatory laboratory testing is necessary to make the diagnosis if history suggests superwarfarin exposure.
- Can consider sending a laboratory test to assess for superwarfarin exposure if patient without known exposure develops persistent unexplained INR elevation.
- This is a send out test that takes several days to weeks to result.
- Can consider sending a laboratory test to assess for superwarfarin exposure if patient without known exposure develops persistent unexplained INR elevation.
Management:
- Unintentional, acute ingestions
- Asymptomatic patients with acute ingestions (<24 hrs) do not require admission to the hospital.
- With small ingestions (e.g., < 1 mg), patients may be monitored at home for two weeks for signs of bleeding of bruising.
- For larger or unknown ingestions, patients should have PT/INR at 48-72 hrs with their primary care physician to further dictate management.
- Report should be made to local poison control center (1-800-222-1222) for follow-up.
- Intentional or chronic ingestions
- Require initial PT/INR and evaluation for co-ingestants (e.g., acetaminophen concentration).
- Charcoal may be useful if administered to prevent absorption in the first 1-2 hours after ingestion.
- Whole bowel irrigation may be considered for an acute massive ingestion or ingestion of intact packets of bait.
- Use the INR to guide management.
- Vitamin K1 should not be administered empirically but only in response to elevated INR.
- If given prior to detecting an elevated INR, vitamin K1 may delay INR elevation and underestimate severity of exposure.9
- If INR is normal at 48-72 hrs, clinical toxicity can be safely ruled out with very rare exceptions.9
- For INR > 2.0 with minor or no bleeding, initiate oral vitamin K1 10-50 mg every 6-12 hours, which may be rapidly titrated up to 50-100 mg every 6 hours. Dosing for children should be 0.4 mg/kg/dose every 6-12 hours.9
- After INR normalizes, vitamin K1 dosing may be titrated down.
- Large superwarfarin ingestions may require outpatient treatment for several months.
- Vitamin K1 should not be administered empirically but only in response to elevated INR.
- For patients with major bleeding
- Blood products should be administered in a similar fashion to serious bleeding with warfarin, including protein complex concentrate, fresh frozen plasma, and packed red blood cells.
- Oral vitamin K1 should be administered as above, unless the patient is critically ill, in which case IV vitamin K1 at a dose of 0.3 mg/kg (max 10 mg) should be administered initially. This carries a risk of anaphylactoid reaction.10 Continued oral vitamin K1 will be required after initial IV dose(s).
Case Follow-up:
The patient’s blood pressure normalized after 1L of crystalloid. His EKG had normal intervals, and acetaminophen and salicylate concentrations were undetectable. Prothrombin time resulted >100 seconds, with INR 12.7, and partial thromboplastin time was 79 seconds. He remained without symptoms. He was administered oral vitamin K1 40 mg every 6 hours. His INR decreased to 1.9 after 3 days of therapy, and he was continued on oral vitamin K1 titrated to an INR of <2 for the next several months.
Clinical Pearls:
- Superwarfarins are extremely potent and long-lasting inhibitors of vitamin K epoxide reductase.
- Most accidental ingestions do not cause significant injury, including in pediatric ingestions.
- Normal INR at 48-72 hours can reliably rule out clinical toxicity with very rare exceptions.
- Vitamin K1 is the mainstay of treatment but should not be started empirically in a stable patient, only in response to INR elevation.
- Resuscitation of serious bleeding is not different from warfarin-induced coagulopathy, comprising blood products in addition to IV vitamin K.
- Patients often need PO vitamin K1 supplementation for months after large ingestion.
- While unnecessary if the history is clear, can consider sending superwarfarin panel to make the diagnosis in unexplained INR elevation.
References:
- Chong YK and T Wing-Lai Mak. Superwarfarin (Long-Acting Anticoagulant Rodenticides) Poisoning: from Pathophysiology to Laboratory-Guided Clinical Management. Clin Biochem Rev. 2019 Nov; 40(4): 175–185.
- Feinstein et al. The emerging threat of superwarfarins: history, detection, mechanisms, and countermeasures. Ann N Y Acad Sci. 2016 Jun; 1374(1): 111–122.
- Vandernbrouke V, Bousquet-Melou A, De Backer P, and Croubles S. Pharmacokinetics of eight anticoagulant rodenticides in mice after single oral administration. J. Vet Pharmacol Therap. 2008 31:437-445.
- Yip L, Stanton NV, Middleberg RA. Vitamin K1 treatment duration in patients with brodifacoum poisoning. N Engl J Med 2020; 382:1764-1765.
- Holford NH. Clinical pharmacokinetics and pharmacodynamics of warfarin. Understanding the dose-effect relationship. Clin Pharmacokinet. Nov-Dec 1986; 11(6): 483-504.
- EPA Takes Major Actions to Reduce Americans’ Risks from Mouse and Rat Poisons / Move will better protect children, pets and wildlife. U.S. Environmental Protection Agency, 2011. Available at: https://archive.epa.gov/epapages/newsroom_archive/newsreleases/5689a230c1490219852578a80053a4b7.html. [Accessed May 18, 2022]
- Caravati EM, Erdman AR, Scharman EJ, et al. Long-acting anticoagulant rodenticide poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicology 2007;45(1):1-22.
- Kelkar et al. An Outbreak of Synthetic Cannabinoid–Associated Coagulopathy in Illinois. N Engl J Med 2018; 379:1216-1223.
- Recognition and management of pesticide poisonings, 6th U.S. Environmental Protection Agency, 2013. Chapter 18: Rodenticides. Available at: https://www.epa.gov/pesticide-worker-safety/recognition-and-management-pesticide-poisonings. [Accessed July 16, 2021.]
- Britt RB and JN Brown. Characterizing the severe reactions of parenteral vitamin K1. Clinical and Applied Thrombosis/Hemostasis 2018, Vol. 24(1) 5-12.








