Authors: Casey Thompson, BS (UK College of Medicine); Amber Brooks, DO (EM Resident Physician, UK Dept of EM); and Chris Belcher, MD (Assistant Professor, UK Dept of EM) // Reviewed by: Marina Boushra, MD (CCM fellow, Cleveland Clinic Foundation); Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)
Case 1
A 71-year-old woman presents to the ED with her son after a forward fall where she hit her chin against a table. She denies any relevant past medical history and notes pain in her neck and chin. On neurologic assessment, she has significantly decreased bilateral handgrip strength and upper extremity strength. She has decreased pinprick sensation on her shoulders, upper back, and posterior arms bilaterally. She has slightly decreased lower extremity strength testing but normal lower extremity pain sensation. She has normal proprioception and normal vibratory sensation of her upper and lower extremities. The remainder of her physical exam is normal with the exception of bruising and tenderness along her chin.
What is the diagnosis? What imaging modality will best evaluate the cause of her symptoms?
Background
Spinal cord injury (SCI) is a common cause of presentation to the emergency department (ED), with an annual incidence of ~18,000 new cases in the United States per year, with males making up 78% of new SCI cases since 2015 (2). The most common cause of acute SCI is motor vehicle accidents, with the next common causes being falls and acts of violence, respectively (1). Of note, about a quarter of SCI involves alcohol (15). In addition, 70-80% of patients with SCI have multisystem trauma, further complicating diagnosis and care of these patients (5, 6). Cervical spinal injuries represent about 60% of all SCI and commonly result in complete or incomplete tetraplegia (2). Although uncommon, pediatric patients are also affected by SCI and represent up to 5% of total traumatic SCI (9).
Evaluation
Due to the high impact causes of most spinal cord injuries, many patients present to the ED via Emergency Medical Services (EMS). Immobilization of anyone at risk for an SCI has been standard practice since the 1970s, despite the lack of evidence-based studies on its efficacy. There are known harms of immobilization, most notably airway compromise, aspiration, and ulcers from prolonged stays in immobilization devices (1). Despite controversy surrounding immobilization devices, they remain in widespread use due largely to the historical and legal precedent. In fact, no high-quality evidence supports use of these immobilization devices (2). Treatment recommendations include expediting the removal of immobilization instruments when possible (1).
A thorough examination of patients with possible SCI is vital to their short term and long-term care, especially considering that as much as 9% of spinal injuries can be missed on initial assessment (2). A thorough history needs to be sought out by the physician from patient and available collateral should be obtained from EMS or witnesses. Inquiry should be made regarding potential or witnessed neurological deficits, with specific attention to motor function. Mechanism of injury provides insight to help build the differential (2). If other more life threatening or operative injuries are found, these should be cared for per the usual institutional trauma guidelines (6).
On initial evaluation of potential SCI, it is prudent to evaluate the usual trauma ABCDEs. Airway can be compromised in cervical spine trauma due to cranial injuries and in-line stabilization should be utilized for airway maneuvers. Early intubation and ventilation may be indicated for patients with high cervical injuries, particularly those at C3, 4, or 5 as these injuries can compromise the diaphragmatic innervation (C3, 4, 5 keep the diaphragm alive!) (2, 5). Although it may worsen laryngoscope view, in-line stabilization remains the standard during intubation of a patient with suspected SCI as there is evidence suggesting worsened neurologic injuries in SCI patients intubated without in-line stabilization (2). These intubations should be done under careful guidance due to this decreased view. A recent review suggests that video laryngoscopy has superior fast-pass success in patients with SCI (16). Fiberoptic intubation may also be considered. Breathing and respiratory complications are the most common cause of morbidity and mortality following SCI, as these patients can have hypoventilation, hypercapnia, hypoxia, and impaired secretion clearance. Therefore, it is vital that the emergency physician vigilantly monitor the patient’s respiratory status (2, 5). Circulation is important as SCI can cause neurogenic or spinal shock resulting in loss of vasomotor tone and cardiac output, which is further expounded upon below (2).
Once ABCs have been evaluated, assessment of Disability via an initial neurological assessment should be obtained during the resuscitative phase to assess level of consciousness and motor function. Finally, proper Exposure is necessary to completely evaluate for further injuries (2). Complete physical exam with a detailed neurological assessment should not be delayed for imaging studies, as this slows recognition of SCI and establishment of baseline function, which has implications for immediate and continued care of patients with SCI (6).
In addition to a global neurologic disability score (Glasgow Coma Scale), the ED physician should perform a detailed motor exam testing each major muscle group in isolation, light touch sensory exam and assessment of rectal tone (6, 4). During this secondary survey, the patient should be log-rolled and the spine palpated for signs or injury, such as step-offs or spinal tenderness. Maintaining in-line stabilization is critical if C-spine injury is suspected (1, 2). A common and well-studied system to score and evaluate this neurologic examination is the American Spinal Injury Association (ASIA) scoring system (Figures 1 and 2), which provides a standardized way of assessing motor and sensory deficits.

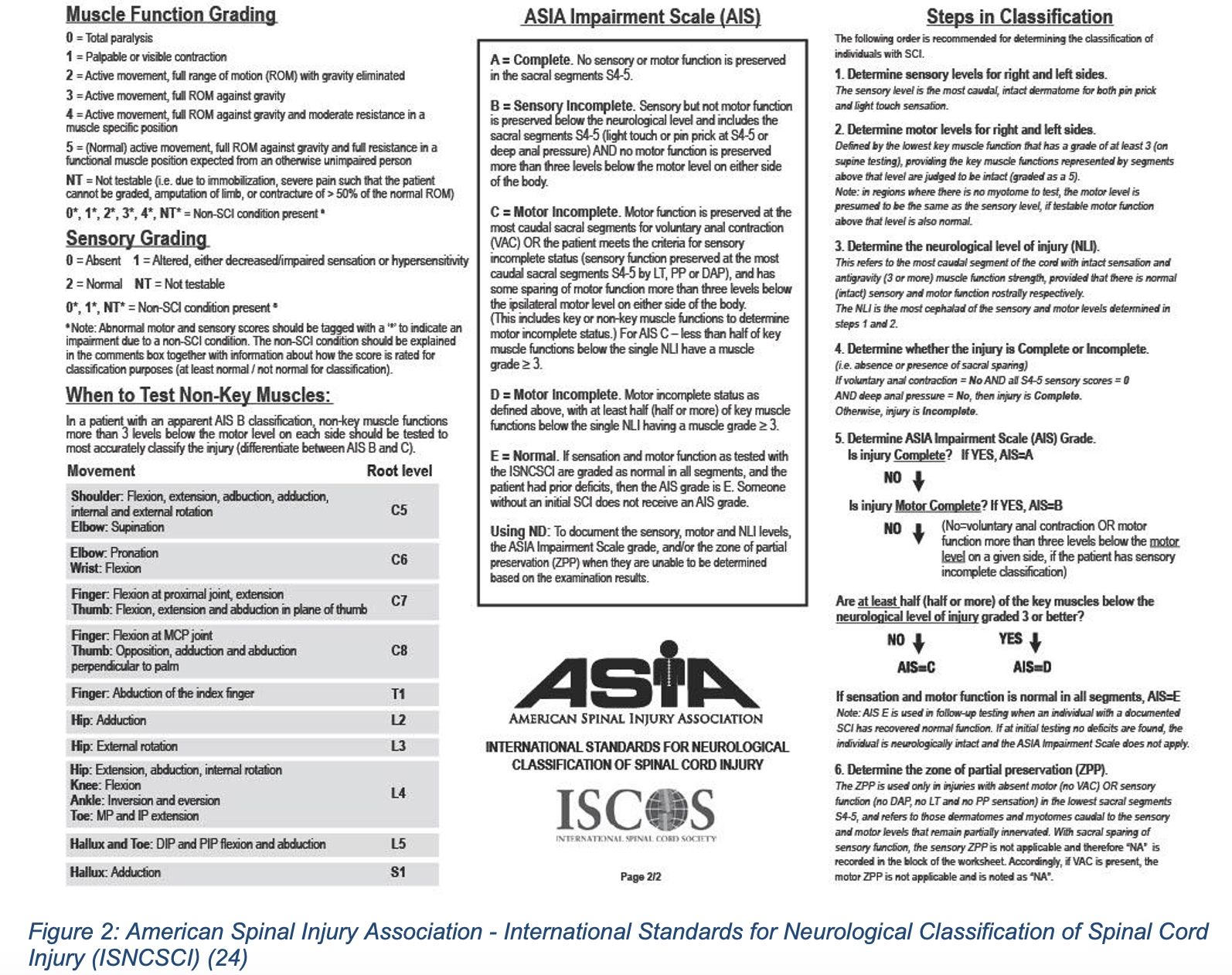
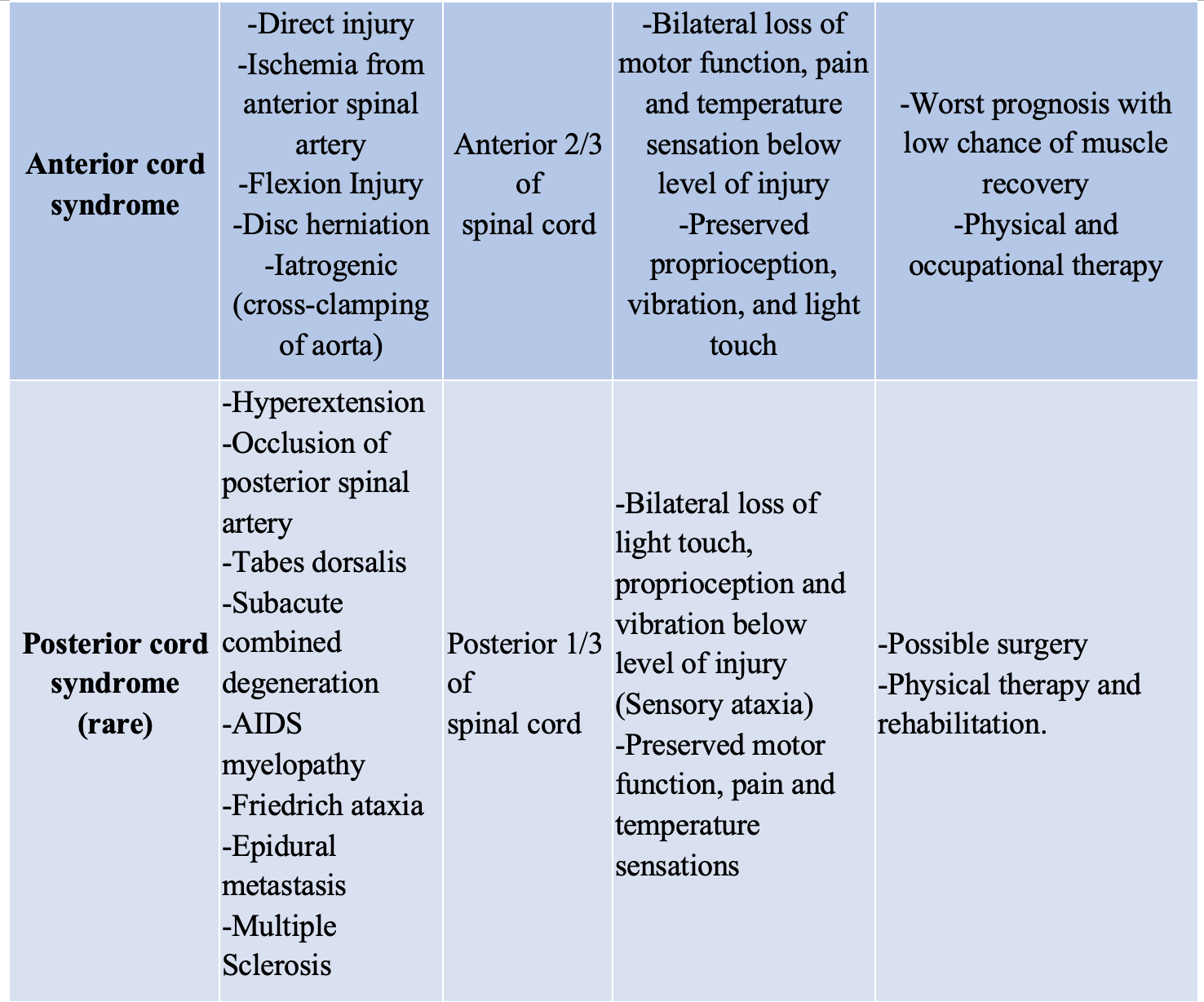
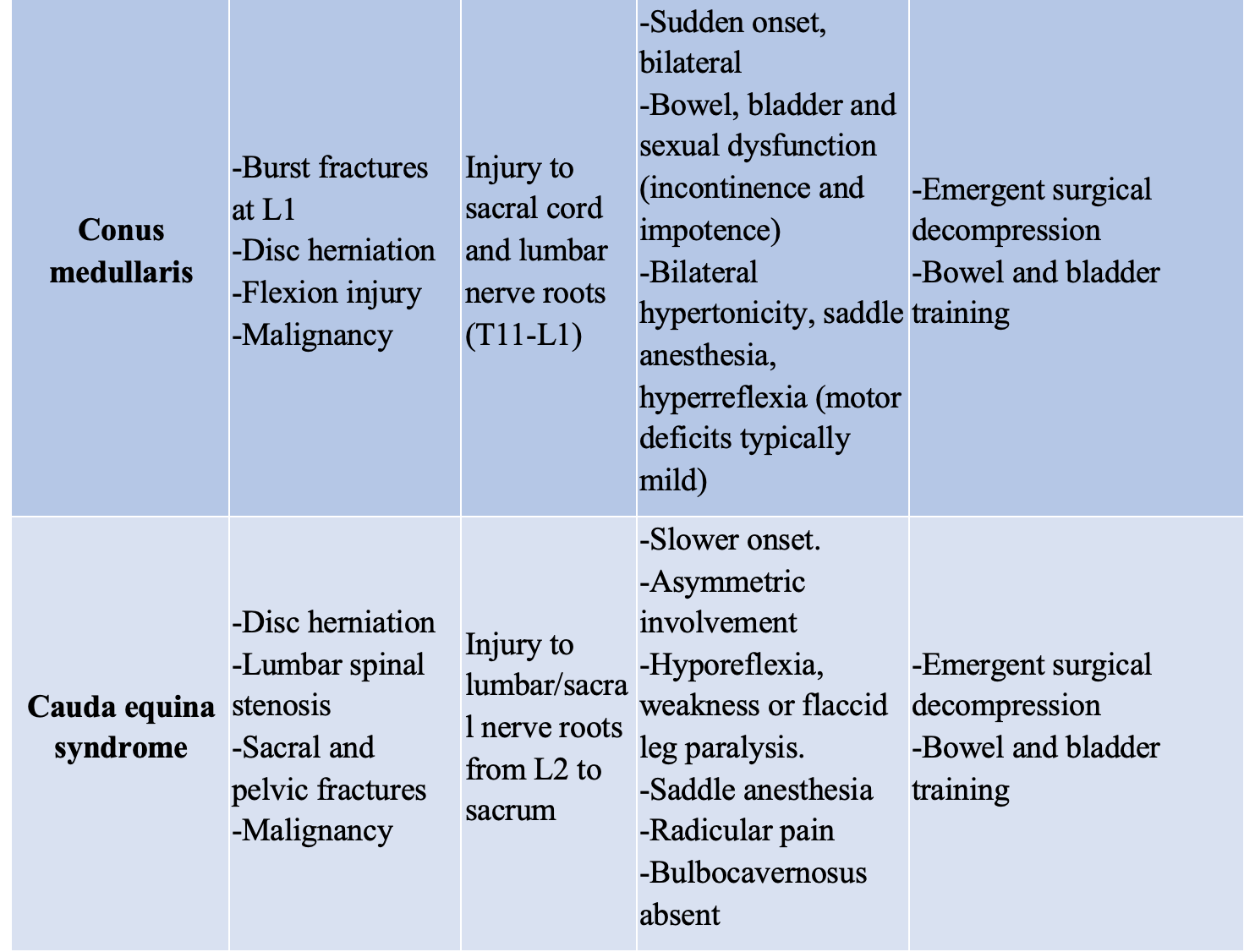
While allowing for a baseline functioning test, this neurologic evaluation performed in the ED can also help to localize a lesion and narrow the differential based on classic presentations such as the complete and incomplete syndromes, summarized below (Table 1).


Imaging for bony cervical injury in the ED is standard in most trauma patients and widely available within the US. However, not every patient scenario necessitates imaging. Currently there are two main decision rules for imaging in patients with SCI, both of which have high sensitivities and specificities for patients with cervical spine injuries. The NEXUS Low Risk criteria (NLC) is a 5-part decision rule that has been largely studied and validated for use in ruling out the necessity of imaging, with a sensitivity of 99%. However, it has a relatively low specificity (12.9%), which means that there are patients without injuries still being imaged from these criteria (1, 3). The other common and validated decision rule is the Canadian C-spine rule (CCR), which was specifically developed to have a greater specificity to limit the unnecessary use of imaging (>99% sensitivity, and ~42% specificity) (1, 3). However, many studies and reviews do not necessarily argue for the use of CCR over NLC as both are very sensitive for cervical SCI, and state if there is any question remaining, then imaging should be done. Currently, there are not widely used or validated decision rules for imaging with thoracolumbar injuries. Though there was one developed in 2015, it is still pending further validation (2). Links to calculators for both criteria are below:
Nexus/NLC: https://www.mdcalc.com/nexus-criteria-c-spine-imaging
CCR: https://www.mdcalc.com/canadian-c-spine-rule
Historically, CCR and NLC were developed when 3-view X-rays were the first line imaging for SCI, but the greater sensitivity and more widespread availability of CT has caused it to replace X-rays in the setting of SCI. CT imaging has a 100% sensitivity with a 99.5% specificity for bony cervical spine injuries, while plain radiographs have only a 45% sensitivity (4). Current guidelines recommend CT in patients who do not meet the CCR or NLC criteria, or all patients with objective neurologic deficits, high-risk injuries, or other high-risk characteristics (3). High risk blunt trauma characteristics would include automobile ejection, death of another passenger in the same vehicle, vehicle roll-over, high-speed (> 40 mph), extrication time > 20 minutes, automobile vs pedestrian at > 5mph, diving injuries, falls > 20 feet for adults, or falls 2-3 or more times the patient’s height for children (25,26). For patients with a negative CT but continued pain or neurologic deficits, MRI has a greater accuracy to evaluate pathologies of the spinal cord, ligamentous, and soft tissue injury (6). MRI is also the gold standard to evaluate active spinal cord compression, vascular injury involving the cord, and provides evidence of traumatic injuries to intervertebral discs and the ligamentous complexes (5).
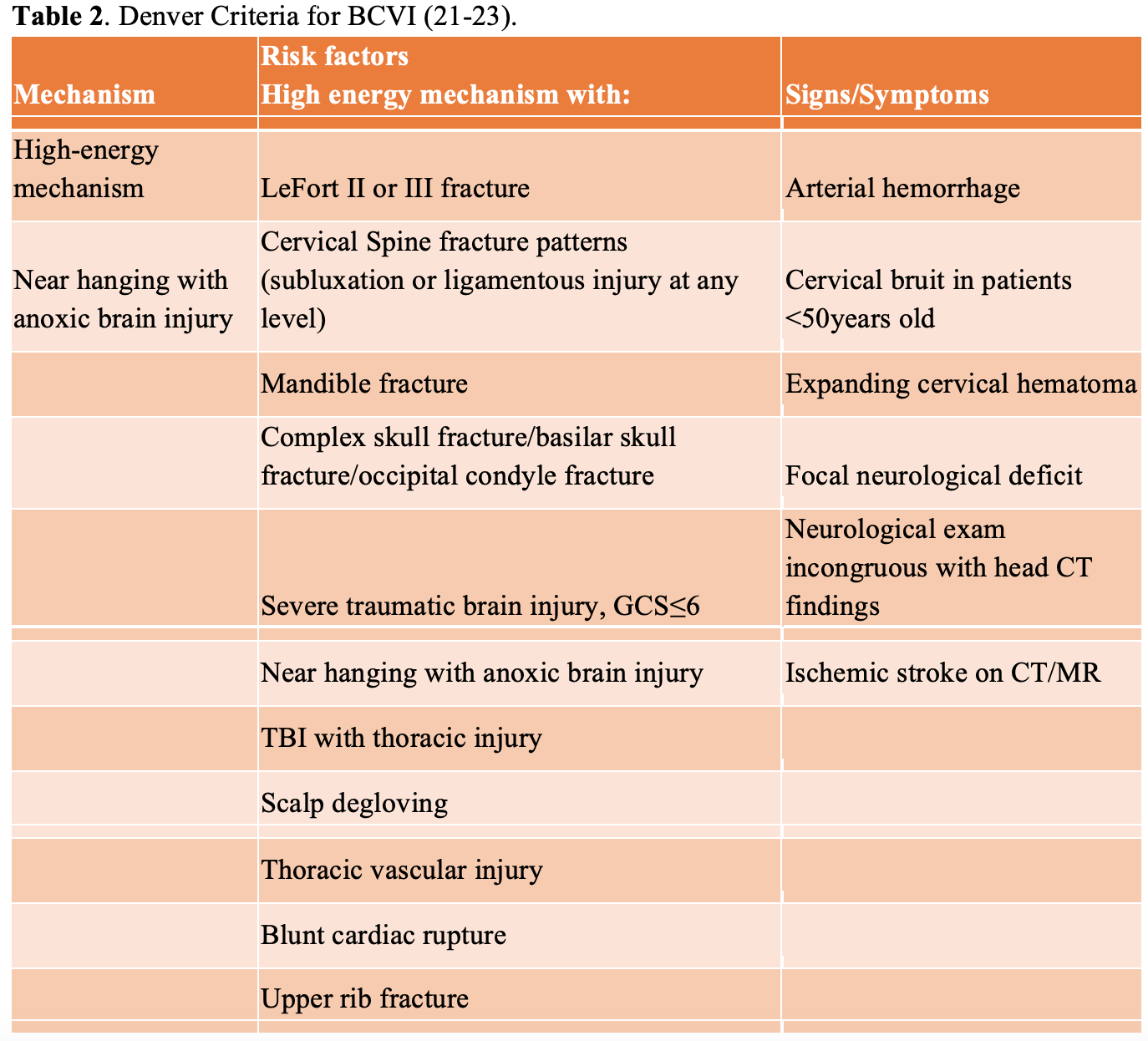
As most spinal cord injuries occur in patients due to high force mechanisms, such as motor vehicle accidents, it is important for the emergency physician to keep a broad differential and be aware of concomitant injuries. One common concomitant injury is blunt cerebrovascular injury (BCVI), which is a non-penetrating injury to the carotid and/or vertebral arteries (23). Patients with these injuries may present with neurological deficits similar to spinal cord injuries. BCVI can be diagnosed via CT angiography (CTA) of the neck, and there are clinical decision instruments available on which patients should receive a CTA. One such clinical decision instrument is the Denver Criteria, which is recommended for use by the Eastern Association for the Surgery of Trauma (EAST) as well as other systematic reviews. These criteria are summarized in the tables 2 and 3 below (21, 22, 23) and should be considered in patients with blunt cervical injury in which SCI is being considered. Of note, current EAST recommendations state that the seat belt sign alone has not been identified as an independent risk factor for BCVI (21).
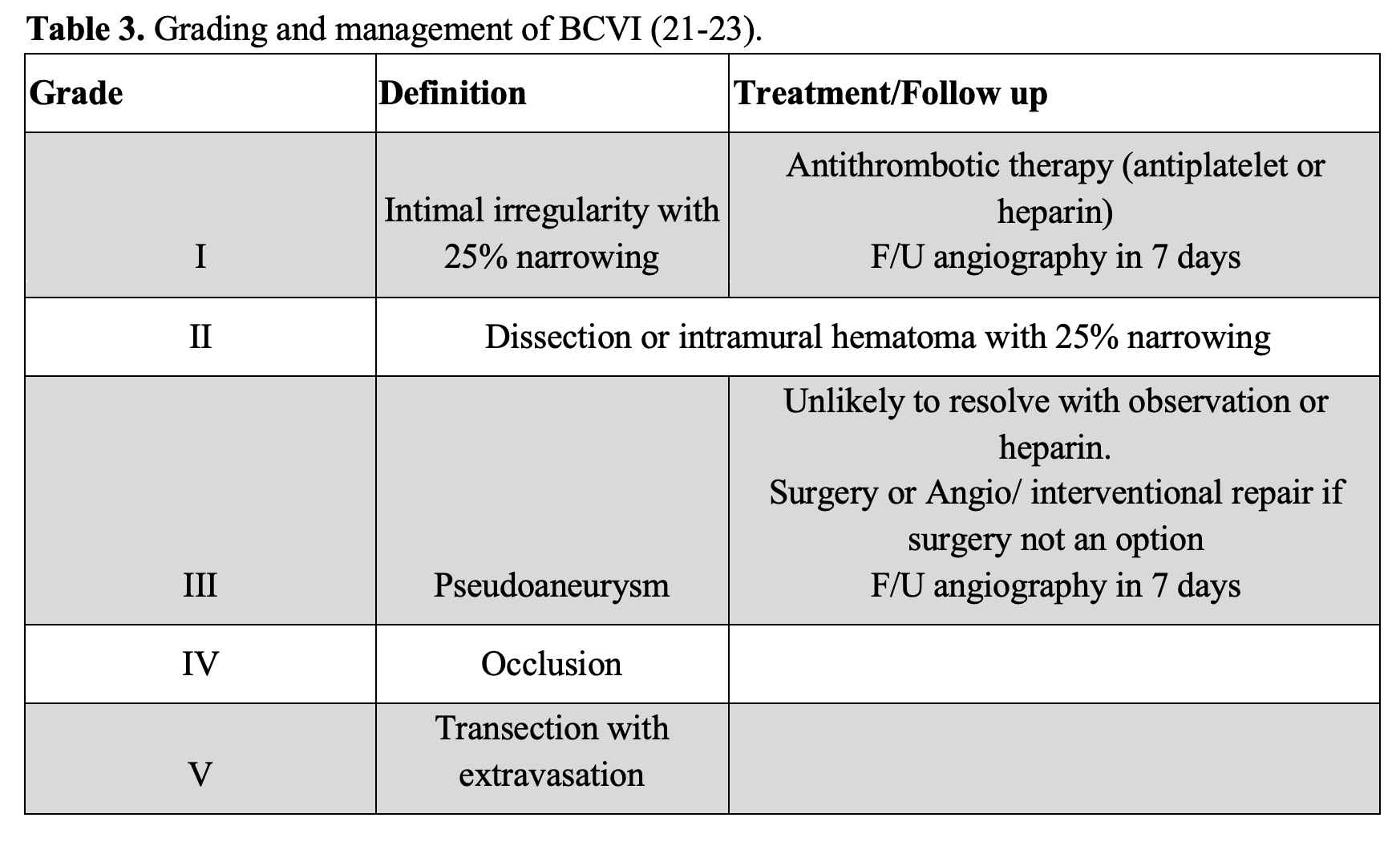
Evaluation of the pediatric patient is also worth noting, since they do present with SCI and can present differently than the adult population. Like the adult population, cervical spine injuries predominate SCI in pediatrics, representing ~80% of injuries (9). Acceleration/deceleration injuries can result in occipito-atlantal and atlantoaxial injuries, and these lesions are worsened by distraction through cervical collar placement and traction. These injuries can be diagnosed via CT and/or MRI and are typically treated conservatively (9). Another SCI in the pediatric population is the atlantoaxial rotatory fixation (AARF, Figure 3), also known as subluxation. It occurs when normal rotation of C1 on C2 becomes fixed. This injury has been linked to minor trauma, pharyngeal infections, and surgery. These patients present with torticollis, with the head turned toward one side and tilted to the other, with an inability to turn the head past midline. A dynamic 3-position CT has a high sensitivity for diagnosing AARF (2).

Figure 3. AARF. Case courtesy of Assoc Prof Frank Gaillard, <a href=”https://radiopaedia.org/?lang=us”>Radiopaedia.org</a>. From the case <a href=”https://radiopaedia.org/cases/8015?lang=us”>rID: 8015</a>
Selected images from an MRI confirm widening of the atlanto-occipital joint with prominent fluid in the prevertebral soft tissues and posterior neck. The cord appears normal and there is no convincing disruption of the theca on these selected images. Superior endplate compression fractures are present in the upper thoracic spine.
Lateral x-ray demonstrates the patient to be intubated. What appears to be a tooth with filling is in the hypopharynx. There is widening and non-congruency of the atlanto-occipital articulation consistent with atlanto-occipital dissociation.
A well-documented and unique injury occurring in the pediatric population is spinal cord injury without radiographic abnormality (SCIWORA), which is defined as “objective signs of myelopathy as a result of trauma” without evidence of fracture or ligamentous instability, excluding MRI (2). Although evidence of injury can be found on MRI, but it was described before MRI studies were widely available. SCIWORA is thought to be from the laxity of pediatric connective tissue, which can allow for pathologic vertebral motion and cord injury without resultant bony injuries or dislocation (2). SCIWORA is most common in younger children and incidence decreases with age, comprising up to 20% of SCI in toddlers 0-3 years old but only ~5% of SCI in teenagers. The most common causes of SCIWORA are motor vehicle crashes and sports injuries, but they can occur with falls from height, being struck by an object, and nonaccidental trauma (10). Children with SCIWORA can have a varied presentation that may include transient paresthesia, motor weakness, sensory loss, sensorimotor defects, abnormal reflexes, abnormal rectal tone, or even as an incomplete cord syndrome described above. Additional injuries to other areas are present in over half of patients with SCIWORA. In the ED, careful neurologic examination is necessary to detect any subtle deficits. Imaging could include radiographs of the lateral cervical spine as well as the cervical spine series or CT imaging, depending on institutional preference. If negative, then MRI should be considered if there is high concern for injury (10). Though this may be an uncommon diagnosis overall, it is still a well-documented and continually studied cause of spinal cord injury within the pediatric population.
While this article has so far focused predominantly on traumatic spinal cord injury, it is important to note that patients can present with non-traumatic spinal cord injury (NTSCI). NTSCI can account for up to 79% of SCI, however, causes of NTSCI vary and can be challenging to diagnose in the emergency setting due to the wide variety of etiologies and non-spinal pathologies that mimic NTSCI (myelopathy mimics). Patients with NTSCI also often present later than traumatic SCI (8). NTSCI can occur from compression, such as epidural abscess, hematoma, tumors or prolapsed intervertebral discs, or from ischemia or inflammation of or near the spinal cord. Physical manifestations of NTSCI vary but acute onset of paraplegia or tetraplegia may indicate acute spinal compression, infarct, or demyelination (17). In one study of NTSCI presentations to the ED, it was found that the predominant cause of NTSCI was spinal metastasis, with inflammatory etiologies coming in second (8). This was a relatively small, single institution study from which it is hard to draw large conclusions, but it highlights the importance of remaining vigilant in the ED as spinal cord injury has many causes.
Case 1 resolution
After a thorough history and physical and the appropriate imaging studies, the patient is diagnosed with canal stenosis secondary to an osteophyte and ossifications. No fracture is seen on CT (Figure 4). Given the high concern for central cord syndrome, neurosurgery is consulted who recommends an MRI, continued cervical spine immobilization, and supportive treatment in an ICU setting. The MRI (Figure 4) confirms the diagnosis of central cord syndrome, and the patient has improvement during their stay with supportive care and physical therapy.
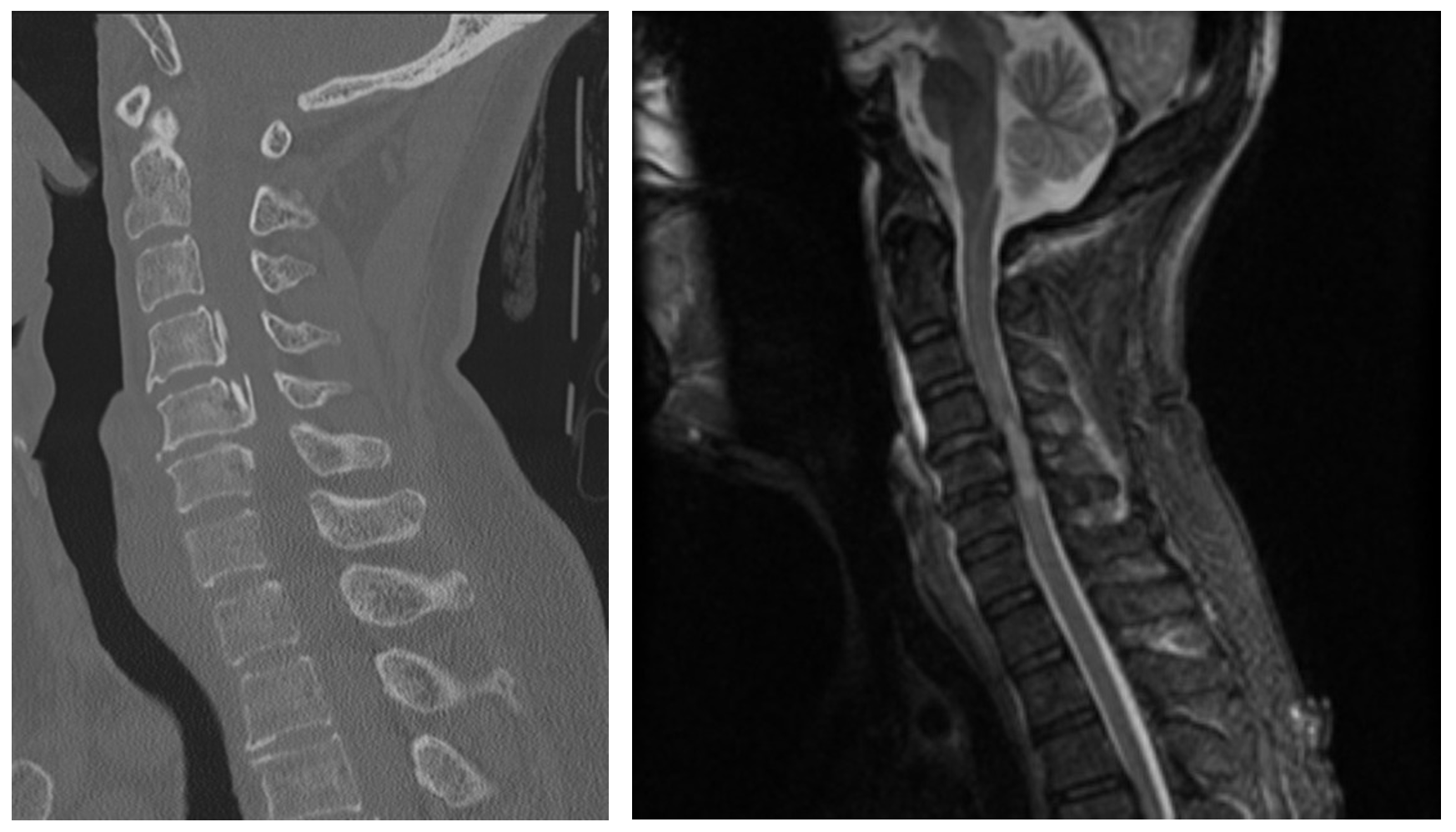
Figure 4. Central Cord Syndrome selected images. Case courtesy of Dr Henry Knipe, <ahref=”https://radiopaedia.org/?lang=us”>Radiopaedia.org</a>. From the case <a href=”https://radiopaedia.org/cases/39562?lang=us”>rID: 39562</a>
CT: No fracture or malalignment. No prevertebral soft tissue swelling. Posterior osteophytes and posterior longitudinal ligament ossification at the level of C5 results in severe canal stenosis.
MRI: Marked central canal stenosis at C5 and C6 secondary to OPLL. There is severe cord compression with high T2 signal from C4/5 to C6. No cord hemorrhage. High T2 signal is also demonstrated within the interspinous ligaments particularly at C4/5, C5/6 and C6/7, with a small amount of abnormal T2 signal also seen in the prevertebral soft tissues. No fractures or convincing disc injury is evident. The anterior longitudinal ligament, ligamentum flavum, and supraspinous ligament/nuchal ligament appear intact.
Case 2
A 28-year-old man with no significant past medical history presents after an altercation. EMS was called to the scene where the patient reported that he had been stabbed in the back by a stranger as he attempted to run away. In addition to severe pain at the site, the patient complains of an inability to move his right leg. Following completion of the primary survey, the patient is noted to have a stab wound lateral to the lower thoracic spine, just to the right of midline. Neurologic assessment demonstrates paralysis and decreased sensation to light touch of the right lower extremity (RLE). He also does not have any discomfort noted with painful stimulus to his left lower extremity, though he does complain of pain when his nailbed on his RLE is pressed. The remainder of his physical exam is unremarkable.
What is the diagnosis? What are the next steps in management?
Management
After initial evaluation and stabilization, early spinal cord injury management is centered around supportive therapy with the goal of minimizing lasting neurologic deficits. Early surgical consultation is paramount. Studies have shown that early surgical decompression, defined as intervention within 8 hours, improves long-term clinical outcomes (11,12). Earlier surgery for these patients has been correlated with a higher rate of conversion from complete to incomplete SCI, with improved outcomes, especially for those who received surgery <12hrs from time of injury (7). Other areas of targeted therapy for the emergency physician specifically center around hemodynamic and, if necessary, respiratory support. Management of other traumatic injuries identified through trauma assessment is also crucial, as traumatic SCI is rarely an isolated injury.
Neurogenic shock is an important consideration in patients with traumatic SCI, which manifests as hypotension, bradycardia, and temperature dysregulation. This is a result of peripheral vasodilation secondary to loss of sympathetic tone with preserved parasympathetic function, and it typically occurs in cervical and upper-thoracic spinal cord injuries. While hemorrhagic shock is a common presentation in trauma patients, key differences include bradycardia, as opposed to tachycardia, and lack of response to volume resuscitation. Neurogenic shock is not synonymous with spinal shock, as spinal shock is loss of muscle tone and reflexes following an injury that typically resolves (20).
In patients with traumatic SCI, AANS recommends maintaining mean arterial blood pressure in the 85-90 mmHg range for approximately 5-7 days post-injury (19). According to a systematic review of the literature regarding MAP management in SCI published by the Journal of Neurosurgery in 2017, norepinephrine is recommended in cervical and upper-thoracic spine injuries, and phenylephrine or norepinephrine is recommended in mid to lower-thoracic injuries (19). The reviewed literature demonstrated stable to improved neurologic outcomes with strict blood pressure management (19).
As mentioned, respiratory support can be a vital part of caring for patients with spinal cord injury, especially considering that pulmonary complications are short- and long-term causes of morbidity and mortality after SCI. Early intubation should be considered for patients with injuries from C1-5, as those are more commonly associated with impaired diaphragm function, respiratory depression, and hypercapnia (5).
Steroids remain a topic of controversy in management of patients with acute SCI. In a systematic review from 2012, patients were found to have improved outcomes at one year with high-dose steroid administration within 3 hours of injury (13). A bolus dose of 30 mg/kg of methylprednisolone over 15 minutes was recommended in these cases, along with a maintenance infusion of 5.4 mg/kg/h infused for 23 hours. They found no evidence for any increased risk of gastrointestinal hemorrhage, wound infections, pulmonary emboli, avascular necrosis, or death (13). Current Emergency Medicine guidelines are based upon the Canadian Association of Emergency Physicians recommendation that state there is insufficient evidence to recommend glucocorticoids as a standard treatment, and there is only weak clinical evidence to suggest glucocorticoids as an option (15). More recent literature recommends against the use of steroids. The American Association of Neurological Surgeons released Level 1 recommendations against the use of steroids within 24-48 hours of SCI in 2013, which remain the most current guidelines. Studies at this time found patients who received IV methylprednisolone had an increased risk of gastrointestinal hemorrhage, wound infection, pulmonary embolism, and thrombophlebitis (14).
Case 2 resolution
Trauma CT scans are obtained showing no bony injury but given the neurological findings, a stat MRI is obtained along with neurosurgical consult. The patient is found to have hemi section of his spinal cord at the level of T11, known as Brown Sequard syndrome. An example MRI of a patient with Brown Sequard syndrome is provided in Figure 5 below.
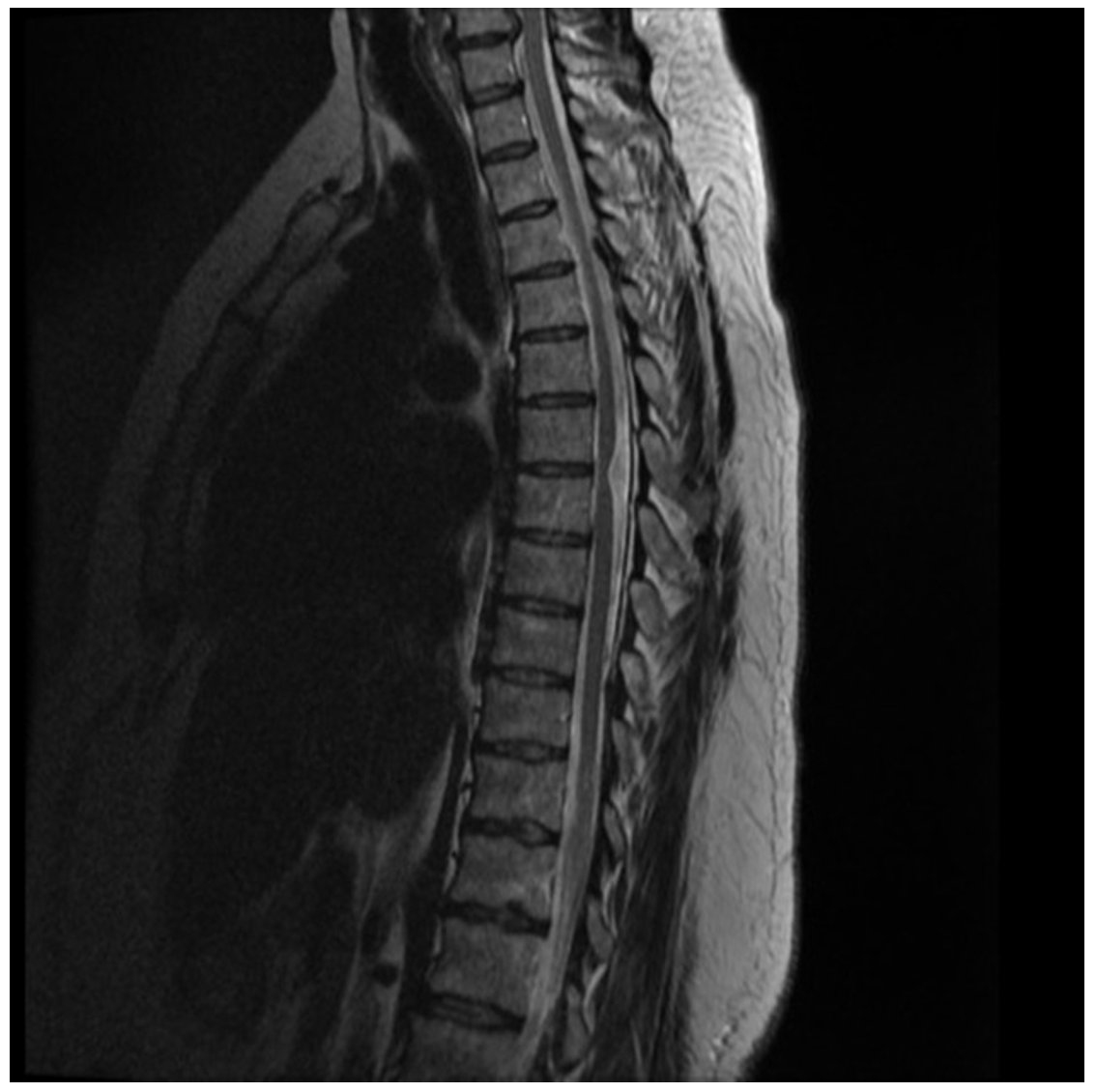
Figure 5. Brown Sequard selected images. Case courtesy of Assoc Prof Frank Gaillard, <ahref=”https://radiopaedia.org/?lang=us”>Radiopaedia.org</a>. From the case <a href=”https://radiopaedia.org/cases/44719?lang=us”>rID: 44719</a>
MRI: At T6/7 the thoracic cord is focally displaced anteriorly and to the right, and markedly distorted without convincing cord signal abnormality. Best seen on sagittal imaging, part of the cord appears to extend beyond the expected location of the theca. Within the CSF space dorsal to the cord, no focal lesion can be identified. No abnormal enhancement. Normal CSF flow voids are noted. Small T12/L1 left paracentral disc protrusion results is no canal stenosis or cord compression. The conus terminates at L1/2 is normal in appearance. All other levels of the thoracic cord are unremarkable. Conclusion: Appearances are characteristic of idiopathic ventral cord herniation at T6/7, presumably due to a congenital dural defect.
The patient is admitted to the hospital and undergoes supportive care along with physical therapy/rehab and has some improvement in his deficits.
Take-away points
-SCI can have devastating consequences contributing to high morbidity in patients and consideration of these pathologies with appropriate neurological exam and imaging should be utilized by the Emergency Physician.
-Although CT should be considered initially for patients with concern for traumatic SCI, MRI is the gold standard for those with neurological deficits localizing to the spinal cord.
-Management hinges on reducing further injury including hemodynamic and respiratory support and early surgical consultation as indicated as earlier surgery has improved outcomes.
References
- LK Beattie and J Choi. Acute Spinal Injuries: Assessment and Management. Emergency Medicine Practice. 8, No. 5; May 2006.
- Jara-Almonte G, Pawar C. Emergency Department Management of Cervical Spine Injuries. Emergency Medicine Practice. Vol. 23, Issue 10; October 2021.
- Gatz J. David, Whetstone William. Spinal Trauma. In: Mattu A and Swadron S, ed. CorePendium. Burbank, CA: CorePendium, LLC. https://www.emrap.org/corependium/chapter/recxnd6zjQJCQ0K5Q/Spinal-Trauma. Updated June 15, 2021
- Shank CD, Walters BC, Hadley MN. Management of acute traumatic spinal cord injuries. In: Handbook of Clinical Neurology. Vol 140. Elsevier B.V.; 2017:275-298.
- Yue JK, Winkler EA, Rick JW, et al. Update on critical care for acute spinal cord injury in the setting of polytrauma. Neurosurgical Focus. 2017;43(5).
- Eckert MJ, Martin MJ. Trauma: Spinal Cord Injury. Surgical Clinics of North America. 2017;97(5):1031-1045.
- Burke JF, Yue JK, Ngwenya LB, et al. Ultra-Early (<12 Hours) Surgery Correlates with Higher Rate of American Spinal Injury Association Impairment Scale Conversion after Cervical Spinal Cord Injury. Neurosurgery. 2019;85(2):199-203.
- Müller-Jensen L, Ploner CJ, Kroneberg D, Schmidt WU. Clinical Presentation and Causes of Non-traumatic Spinal Cord Injury: An Observational Study in Emergency Patients. Frontiers in Neurology. 2021;12.
- Benmelouka A, Shamseldin LS, Nourelden AZ, Negida A. A Review on the Etiology and Management of Pediatric Traumatic Spinal Cord Injuries. Advanced journal of emergency medicine. 2019;4(2):e28.
- Farrell CA, Hannon M, Lee LK. Pediatric spinal cord injury without radiographic abnormality in the era of advanced imaging. Current Opinion in Pediatrics. 2017;29(3):286-290.
- Grassner L, Wutte C, Klein B, et al. Early decompression (<8h) after traumatic cervical spinal cord injury improves functional outcome as assessed by spinal cord independence measure after one year. J Neurotrauma 2016;33:1658-66.
- Lee DY, Park YJ, Song SY, et al. The importance of early surgical decompression for acute traumatic spinal cord injury. Clin Orthop Surg 2018;10:448-54.
- Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2012;1(1):CD001046. Published 2012 Jan 18. doi:10.1002/14651858.CD001046.pub2
- Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery. 2013;7 Suppl 2:93-105.
- Sweis R, Biller J. Systemic Complications of Spinal Cord Injury. Current Neurology and Neuroscience Reports. 2017;17(2).
- Cabrini L, Baiardo Redaelli M, Filippini M, et al. Tracheal intubation in patients at risk for cervical spinal cord injury: A systematic review. Acta Anaesthesiologica Scandinavica. 2020;64(4):443-454.
- Caulfield AF, Flower O, Pineda JA, Uddin S. Emergency Neurological Life Support: Acute Non-traumatic Weakness. Neurocritical Care. 2017;27:29-50.
- Hugenholtz H, Cass DE, Dvorak MF, Fewer DH, Fox RJ, Izukawa DM, Lexchin J, Tuli S, Bharatwal N, Short C. High-dose methylprednisolone for acute closed spinal cord injury – only a treatment option. Can J Neurol Sci. 2002 Aug;29(3):227-35. PMID: 12195611.
- Saadeh, Y. S., Smith, B. W., Joseph, J. R., Jaffer, S. Y., Buckingham, M. J., Oppenlander, M. E., Szerlip, N. J., & Park, P. (2017). The impact of blood pressure management after spinal cord injury: a systematic review of the literature, Neurosurgical Focus FOC, 43(5), E20.
- Dave S, Cho JJ. Neurogenic Shock. [Updated 2022 Feb 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459361/
- Bromberg W, Collier B, Diebel L, et al. Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma. 2010;68(2):471-477. https://www.ncbi.nlm.nih.gov/pubmed/20154559
- Biffl W, Cothren C, Moore E, et al. Western Trauma Association critical decisions in trauma: screening for and treatment of blunt cerebrovascular injuries. J Trauma. 2009;67(6):1150-1153. https://www.ncbi.nlm.nih.gov/pubmed/20009659
- Brommeland T, Helseth E, Aarhus M, et al. Best practice guidelines for blunt cerebrovascular injury (BCVI). Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine. 2018;26(1).
- Roberts TT, Leonard GR, Cepela DJ. Classifications In Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin Orthop Relat Res. 2017;475(5):1499-1504.
- Young AJ, Wolfe L, Tinkoff G, Duane TM. Assessing Incidence and Risk Factors of Cervical Spine Injury in Blunt Trauma Patients Using the National Trauma Data Bank. Am Surg. 2015 Sep;81(9):879-83. PMID: 26350665.
- Leonard JC, Browne LR, Ahmad FA, Schwartz H, Wallendorf M, Leonard JR, Lerner EB, Kuppermann N. Cervical Spine Injury Risk Factors in Children With Blunt Trauma. Pediatrics. 2019 Jul;144(1):e20183221. doi: 10.1542/peds.2018-3221. PMID: 31221898; PMCID: PMC6615532.








