Welcome back to the “52 in 52” series. This collection of posts features recently published must-know articles. Our fourteenth post looks at the DOREMI trial.

Author: Christiaan van Nispen, MD (Emergency Medicine Physician Resident, San Antonio, TX) and Brannon Inman (Chief Resident, Emergency Medicine Physician, San Antonio, TX) // Reviewed by: Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)
Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock
AKA: The “DOREMI” Trial
Clinical question:
In the treatment of adults with cardiogenic shock, is milrinone superior to dobutamine in the prevention of the composite incidence of in-hospital all-cause mortality, cardiac arrest requiring resuscitation, receipt of cardiac transplantation or mechanical circulatory support, myocardial infarction, TIA or stroke, or the initiation of renal replacement therapy?
Study design:
Single center, randomized, double-blinded, clinical trial
Note: Unblinded to the registered nurse administering the trial drug
PICO:
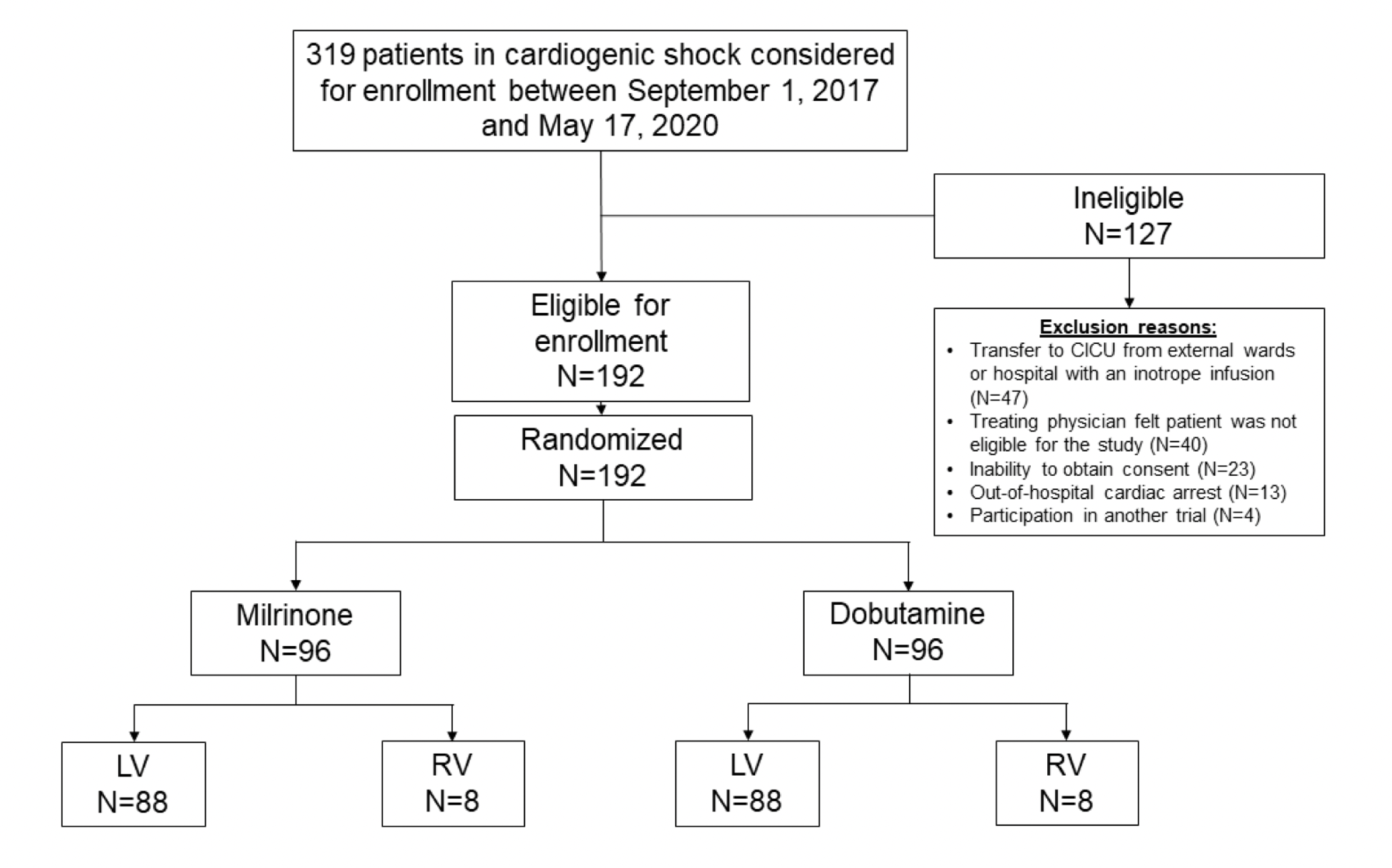
Population:
- 192 adults (of 319 screened) in cardiogenic shock at one quaternary care cardiac institute from 2017-2020
- Included: patients screening positive for at least one of:
- Clinical diagnosis of cardiogenic shock to include systolic blood pressure < 90 mm Hg with evidence of end-organ dysfunction
- Clinical evidence of systemic and/or pulmonary congestion despite the use of vasodilators and/or diuretics
- Acute coronary syndrome complicated by cardiogenic shock with hemodynamic reduction in cardiac index
- Clinically determined to need augmentation of cardiac output in addition to ongoing other vasopressor therapy
- Clinical assessed to require inotropic therapy for developing cardiogenic shock even if no evidence of hypoperfusion
- Excluded:
- Patients who suffered out-of-hospital cardiac arrest
- Pregnant patients
- Milrinone or dobutamine already initiated prior to screening – most common exclusion
- Treating physician opinion that patient should not be in the study – second most frequent cause of exclusion
- Enrolled in another trial
- Balanced groups for the presence of left ventricular versus right ventricular dysfunction
Randomization:
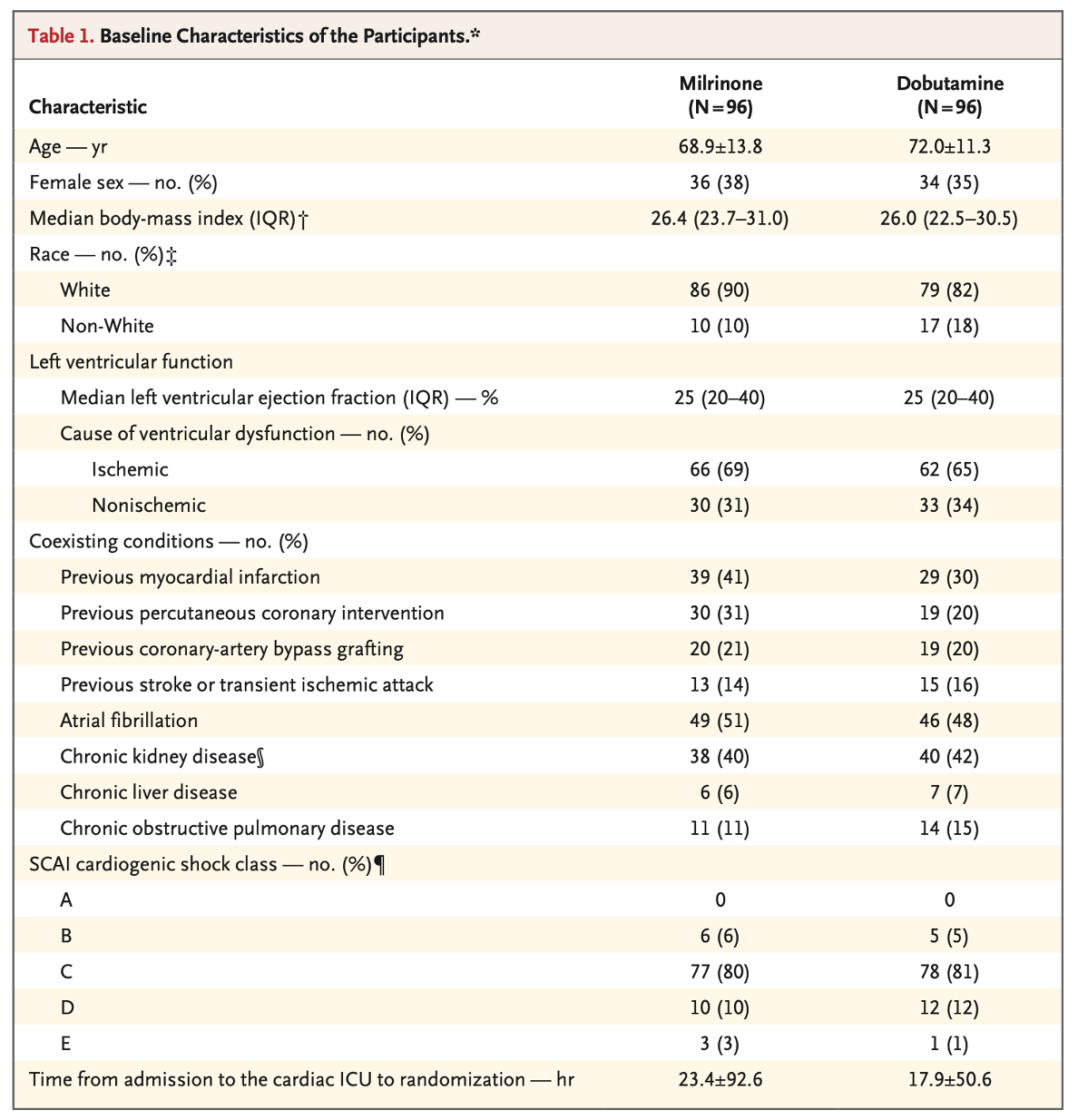
- Groups largely balanced
- Milrinone group with a nonsignificant trend to be younger, more white, more with prior myocardial infarctions, and longer time from admission to randomization (though this could be affected by an outlier)
- Dobutamine group with 50% patients on vasopressors at randomization (39% in milrinone); see table in appendix
Intervention: Milrinone
- Standardized weight-based dosing scale used: 0.125, 0.250, 0.375, 0.500, > 0.500 µg/kg
- The treatment team could request increases or decreases in dose without unblinding
- The treatment team could request unblinding if the team declared it unsafe for blinding to continue
- Compared to dobutamine, researchers estimated that milrinone would produce a 20% lower incidence of the primary outcome, based upon a review of a prior observational study; the DOREMI study powered based on this estimate
Comparator: Dobutamine
- Standardized weight-based dosing scale used: 2.5, 5.0, 7.5, 10.0, > 10.0 µg/kg
- The treatment team could request increases or decreases in dose without unblinding
- The treatment team could request unblinding if the team declared it unsafe for blinding to continue
- Researchers estimated that dobutamine would produce a composite primary outcome in 55% of patients, based upon a review of a prior meta-analysis
Outcome:
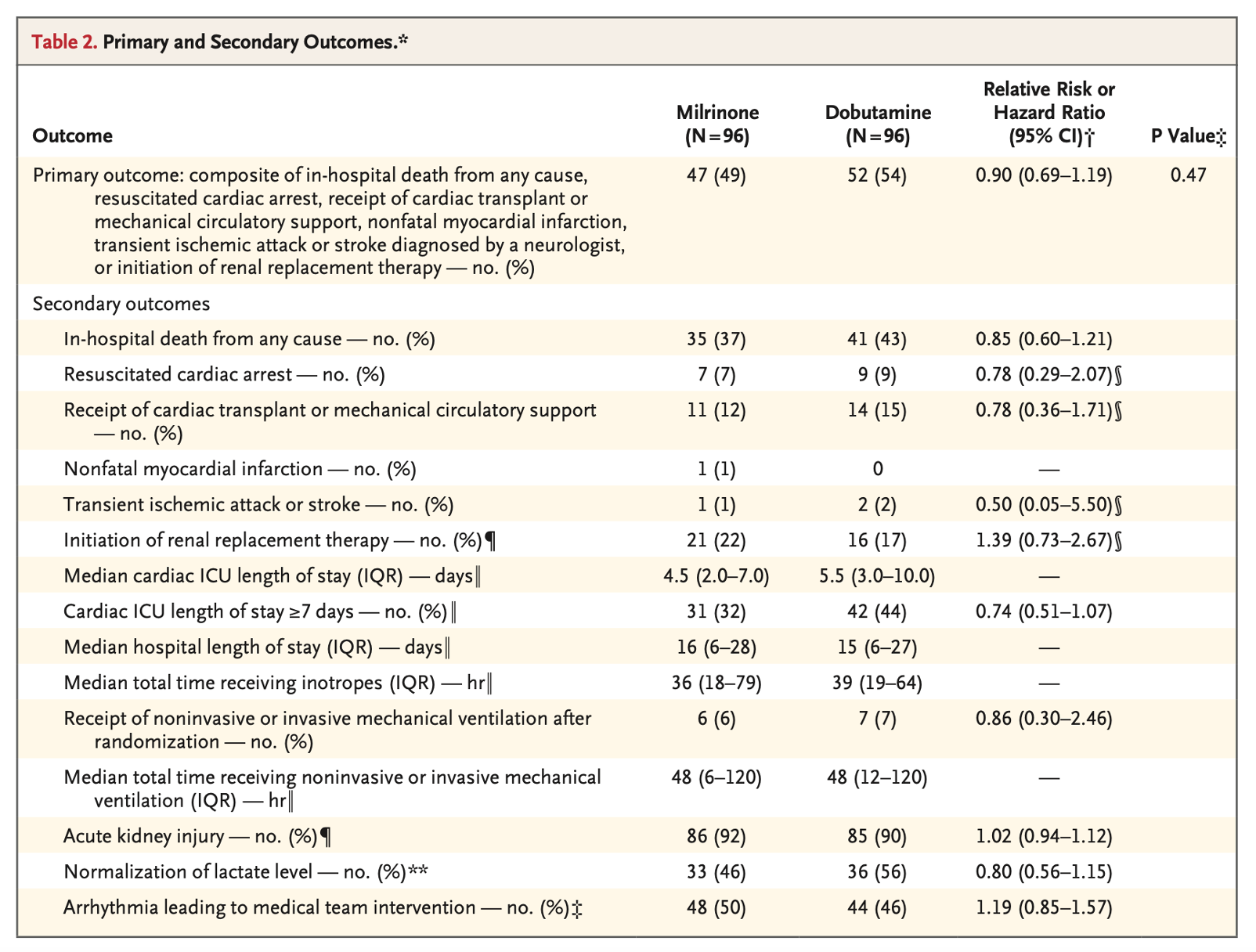
- The primary outcome was a composite of:
- In-hospital all-cause mortality
- Cardiac arrest with resuscitation
- Receipt of cardiac transplantation or mechanical circulatory support
- Nonfatal myocardial infarction
- Transient ischemic attack or stroke diagnosed by a neurologist
- Initiation of renal replacement therapy
- Secondary outcomes:
- Any individual primary outcome, not composite
- Intensive care unit admission ≥ 7 days
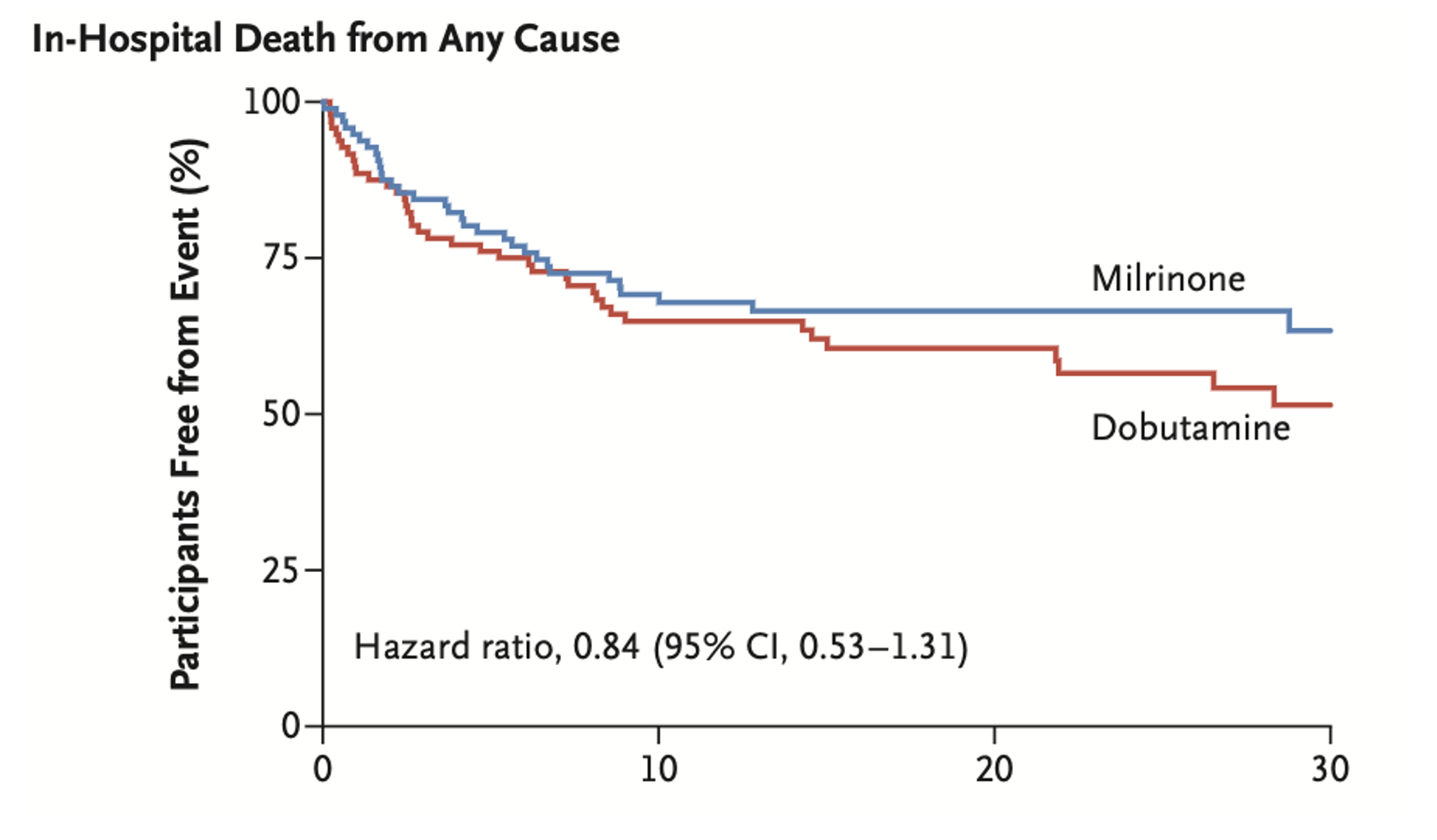
- No statistically significant differences in primary or secondary outcomes
- No statistically significant differences in markers of resuscitation such as lactic acid level or urine output per hour

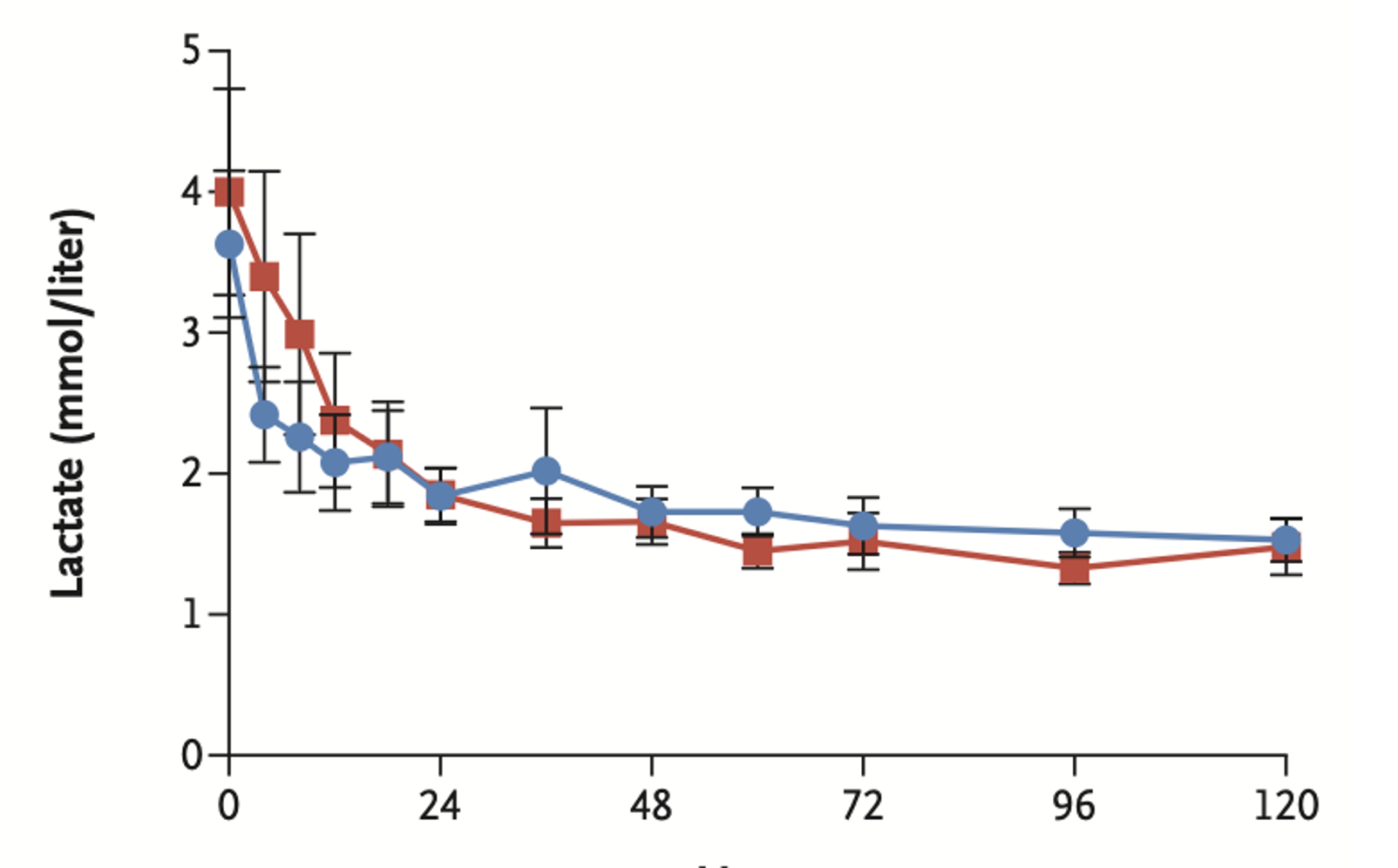
- Note: the study was powered for large effect size (20%)
Take Aways:
- Negative trial
- The study was underpowered to detect smaller effect sizes, so it remains possible that milrinone is superior to dobutamine, or vice-versa.
- Several outcomes of various severity are grouped in the composite primary outcome; it would be far worse to die than have a non-fatal medical complication.
- Although each primary outcome is compared across groups, this study did not have sufficient power to appreciate changes.
- Unclear if a difference exists between their medications at different stages of cardiogenic shock; trial enrolled patients at stages B, C, D, and E according to the definition of the Society of Cardiovascular Angiography and Interventions (SCAI) but did not perform statistical analyses to determine if patients in each subgroup had different outcomes.
- Treating physicians had significant power to influence the study, including which patients could be enrolled, dosing of enrolled patients, and the ability to unblind on demand (unclear how often, if ever, this happened).
My Take:
Compared to milrinone, dobutamine is more often available, and ancillary staff are more familiar with the medication. I will continue to use dobutamine as my initial inotrope of choice from the ED for the management of cardiogenic shock.
Reference:
Mathew R, Di Santo P, Jung RG, et al. Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock. N Engl J Med. 2021;385(6):516-525. doi: 10.1056/NEJMoa2026845.








