Welcome back to the “52 in 52” series. This collection of posts features recently published must-know articles. This week we cover the PREVENT trial. Does low (6 mL/kg) tidal volume reduce ventilator free days in ventilated patients without ARDS compared to intermediate tidal volume (10 mL/kg)?

Author: Brannon Inman (Chief Resident, Emergency Medicine Physician, San Antonio, TX) // Reviewed by: Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)
Effect of a Low vs Intermediate Tidal Volume Strategy on Ventilator-Free Days in Intensive Care Unit Patients Without ARDS
AKA: The “PREVENT” Trial
Clinical question:
Does low (6 mL/kg) tidal volume reduce ventilator free days in ventilated patients without ARDS compared to intermediate tidal volume (10 mL/kg)?
Study design:
- Phase 3, randomized, clinical trial enrolling patients in 6 ICUs in the Netherlands
PICO:
Population:
- Inclusion:
- Adult ICU patients on mechanical ventilation before or after admission to the ICU.
- Patients expected not to be extubated within 24 hours of randomization
- Patients able to be randomized within 1 hour of admission to ICU
- Exclusion:
- The presence of ARDS (according to the criteria of the Berlin criteria)
- Patient < 18 years
- Pregnancy
- Ventilation longer than 12 hours before admission to the ICU
- Increased/uncontrollable intracranial pressure
- History of pulmonary disease
- New pulmonary embolism
- Previously randomized in this trial
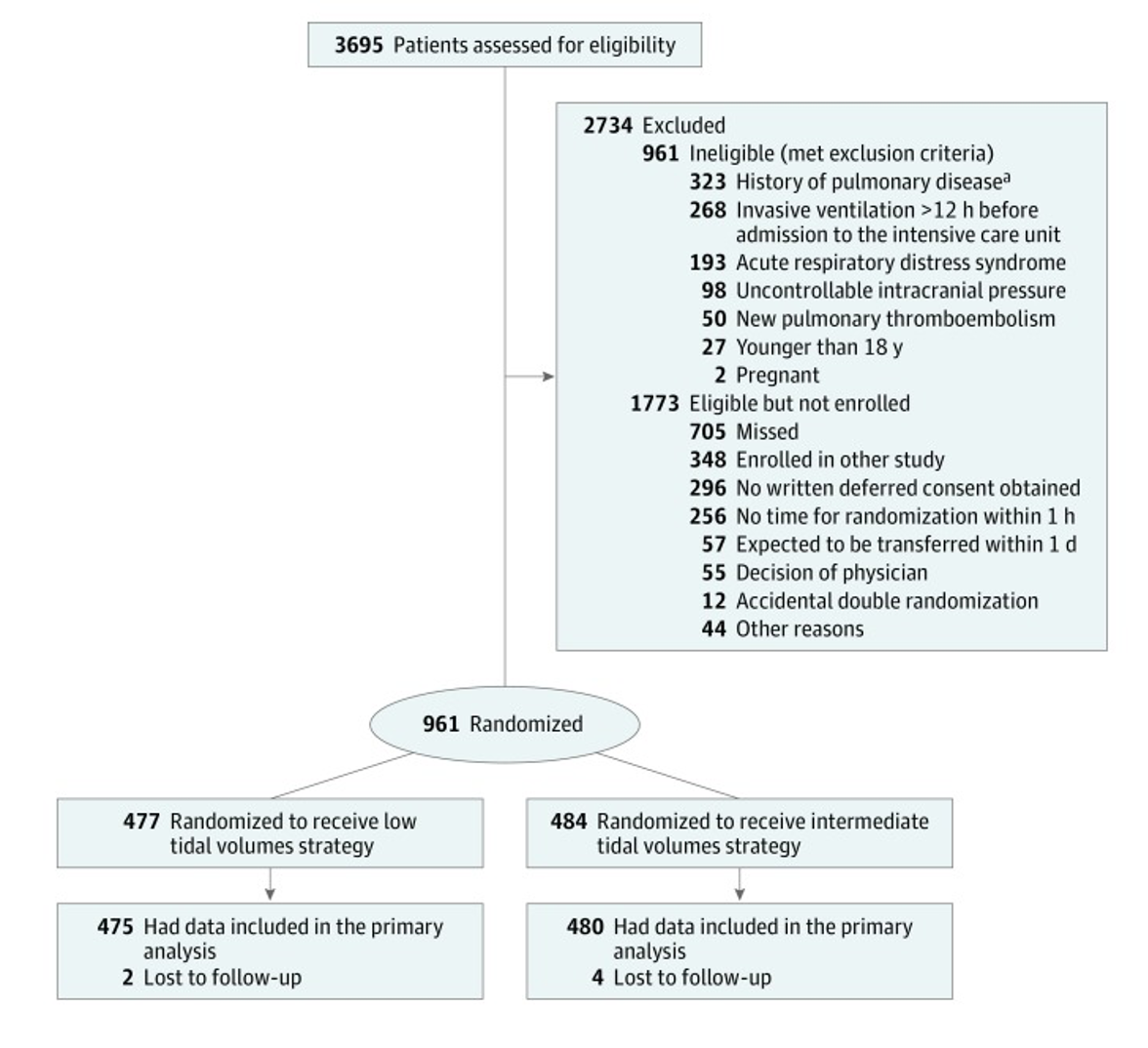
Intervention:
- Patient initiated on 6 mL/kg tidal volume
- Volume control (VC) or pressure control (PC) were allowed
- If VC was used the tidal volume was decreased by 1 mL/kg every hour to a minimum of 4 mL/Kg
- If PC, the minimum pressure was 5 cm H2O
- If tidal volume was >8 mL/Kg despite 5 cm H2O this was tolerated
- Volume control (VC) or pressure control (PC) were allowed
Comparator:
- Patient initiated on 10 mL/kg tidal volume
- Volume control (VC) or pressure control (PC) were allowed
- If VC was used:
- Plateau pressure was monitored and if >25 cm H2O, tidal volume was decreased by 1 mL/kg PBW per hour
- If PC was used:
- Pressure support was adjusted to goal tidal volume while monitoring the plateau pressure keeping the pressure less than 25 cm H2O
- If VC was used:
- Volume control (VC) or pressure control (PC) were allowed
Outcome:
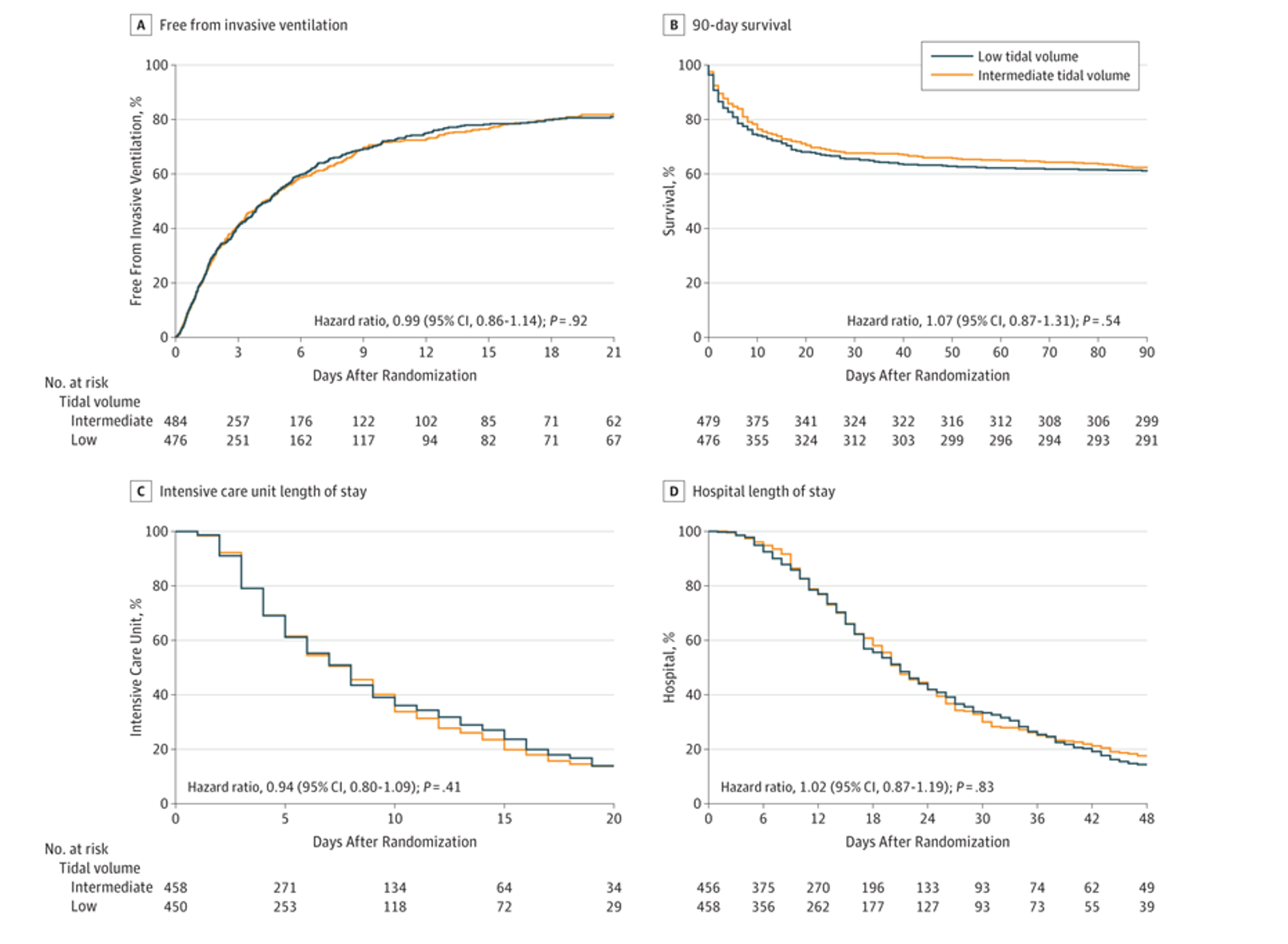
- Primary outcome was ventilator free days and alive at day 28.
- No statistically significant difference in primary outcome between groups.
- Patients in both groups had a median of 21 ventilator-free days 28 days after randomization.
- (IQR, 0-26) (mean difference, –0.27 [95% CI −1.74 to 1.19]; P = .71
- Secondary outcomes included length of ICU and hospital stay, 28 and 90 day mortality in ICU and the hospital, development of ARDS/pneumonia/severe atelectasis/pneumothorax
- No significant difference in secondary outcomes.

Take Aways:
- Negative trial/no difference between 6 mL/kg and 10 mL/kg tidal volumes on ventilator free days (primary outcome) and a number of secondary outcomes (ie, mortality, length of stay, adverse events).
- This was a large trial, with good methodology and pre-published statistical methods.
- Authors used an intention to treat analysis.
- Unfortunately, the study focused on ventilator free days, rather than mortality as a primary outcome. Mortality is an optimal patient centered outcome, rather than ventilator free days. Families probably care if their family member lived, more than they care if they were off the ventilator a few days earlier.
- The study focuses on a relatively simple metric as a surrogate for preventing lung injury. While overlooking more complicated measures, a focus on this discrete variable is of benefit in its application to the ED patient.
My take:
- While I wouldn’t expressly target tidal volumes above ARDSNet guidelines, based on these data, if patients are transiently on tidal volumes of 6-10 mL/kg, I am not convinced there would be harm. Current evidence still compels me to advise erring toward 6mL/Kg.
Source Article:
- Writing Group for the PReVENT Investigators; Simonis FD, Serpa Neto A, Binnekade JM, Braber A, Bruin KCM, Determann RM, Goekoop GJ, Heidt J, Horn J, Innemee G, de Jonge E, Juffermans NP, Spronk PE, Steuten LM, Tuinman PR, de Wilde RBP, Vriends M, Gama de Abreu M, Pelosi P, Schultz MJ. Effect of a Low vs Intermediate Tidal Volume Strategy on Ventilator-Free Days in Intensive Care Unit Patients Without ARDS: A Randomized Clinical Trial. JAMA. 2018 Nov 13;320(18):1872-1880. doi: 10.1001/jama.2018.14280. PMID: 30357256; PMCID: PMC6248136.








