Author: Brit Long, MD (@long_brit) // Reviewed by Alex Koyfman, MD (@EMHighAK)
The American Heart Association (AHA) and Neurocritical Care Society (NCS) released their 2023 Scientific Statement on the critical care management of post ROSC patients. This post covers the key components, with each section starting with the key takeaway followed by the scientific statements.

Methods
This scientific statement was led by the AHA and NCS and focused on the acute and critical care management of adult cardiac arrest survivors of in-hospital and out-of-hospital cardiac arrest. Individual topics were organized by organ system, with small groups addressing each system.
Systems included neurological, cardiac, pulmonary, hematology, infectious disease, gastrointestinal, endocrine, fluids management, and general critical care. The neurologic section was divided into (1) brain oxygenation, perfusion, edema, and intracranial pressure (ICP); (2) seizures and the ictal-interictal continuum (IIC); and (3) sedation and analgesia.
Each small group identified topics, and then the topics were voted on by the entire panel. Topic voted to be the highest priority were evaluated further. The small groups consisted of 3 experts each, which performed a literature search. Each group the developed suggested statements with background and rationale, followed by review of these statements by the panel. Panelists then voted on the statements (“agree” vs. “disagree”). Over 80% of the voting panelists had to be in agreement for inclusion of the statement, with each statement listed with the percentage and the ratio of those voting to include it over the total number of panelists. If > 80% was not achieved, the group could revise/refine the statement. Statements were not included if agreement was not achieved after 3 voting cycles.
Brain Oxygenation, Perfusion, Edema, and ICP Statements
Takeaway: Avoid 100% oxygen saturation. Instead, target 92-98%. Keep hemoglobin > 7 g/dL. Consider lowering ICP if elevated (head of bed elevation, hyperosmolar therapy, etc.).
Statements:
To prevent or treat secondary brain hypoxia in comatose CA survivors, optimize cerebral oxygen delivery by maintaining optimal CPP, arterial normocapnia, and adequate arterial oxygen content while avoiding arterial hyperoxemia (90.5%, 19/21).
To optimize cerebral oxygen delivery in comatose post-CA survivors, maintain hemoglobin >7 g/dL and arterial oxyhemoglobin saturation between 92% and 98% (85.7%, 18/21; see also section on hematologic management).
In comatose CA survivors, continuous monitoring for secondary brain hypoxia may be used in ICUs where validated techniques are in routine use, when there are no contraindications, and when invasive monitoring is consistent with the goals of care (100%, 18/18).
In ICUs where advanced cerebral monitoring is not in routine use, target an MAP >80 mm Hg unless there are clinical concerns or evidence of adverse consequences (82.6%, 19/23).
In ICUs where noninvasive monitoring of cerebral autoregulation is in routine use, maintain MAP at or near the predicted MAPOPT (88.2%, 15/17).
In comatose CA survivors with clinical indicators of cerebral edema and elevated ICP (as measured by head computed tomography, optic nerve ultrasound, or deterioration of clinical examination), consider invasive ICP monitoring in clinical environments familiar with the technique if there are no contraindications and invasive monitoring is consistent with the goals of care (81%, 17/21).
In settings where invasive ICP monitoring is in routine use, maintain MAP at or near the predicted MAPOPT by using the pressure reactivity index (100%, 17/17).
Comatose CA survivors with elevated ICP may benefit from pharmacological and nonpharmacological strategies to lower ICP in the critical care environment (85.7%, 18/21).
EEG Monitoring and Seizures Statements
Takeaway: If possible, obtain an EEG to evaluate for seizure activity. Treat seizures if present. Valproic acid and levetiracetam are reasonable first-line therapies for EEG or clinical seizure activity.
Statements:
Monitor for seizures and SE with EEG as early as possible after CA and during the rewarming phase if temperature control with a hypothermic temperature target is used. Continue EEG monitoring for 72 to 120 hours after CA in patients who fail to recover consciousness. If seizures or SE is diagnosed, the duration and frequency of EEG monitoring are individualized on the basis of treatment goals (85%, 17/20).
Monitor patients who fail to recover consciousness with cEEG to screen for seizures or SE. Intermittent EEG monitoring can be considered as an alternative monitoring modality, depending on the resources of a given institution (100%, 19/19).
In patients undergoing intermittent EEG monitoring, obtain EEGs daily during the first 72 to 120 hours after CA in patients who fail to recover consciousness (90%, 18/20).
Continue cEEG monitoring for at least 24 hours after post-CA seizures or SE initially abate electrographically in patients who fail to recover consciousness because of the possibility of nonconvulsive seizures or SE in this population (100%, 19/19).
Consider transfer to a center that can perform EEG monitoring in patients suitable for transfer who fail to recover consciousness after CA (90%, 18/20).
Consider quantitative EEG trends such as spectrograms and amplitude-integrated EEG as an adjunctive monitoring strategy for seizure screening (84%, 16/19).
Interpret the EEG as soon as possible after the recording is started, and report results rapidly to the team in charge of medical management (95%, 19/20).
Ensure that written EEG reports are updated at least daily and are available to the team in charge of medical management (90%, 18/20).
Consider the clinical context of patient management in the interpretation of EEG and written report of EEG findings, including factors such as clinical examination, use of sedatives and ASMs, and hemodynamic and metabolic factors (95%, 19/20).
Follow the same treatment standards used for other causes of seizures or SE in patients with post-CA seizures or SE, assuming that the goals of care are compatible with aggressive treatment (95%, 19/20).
Evaluate and treat seizures or SE after CA in the context of other available clinical information because other systemic factors may influence the occurrence of seizures or SE and the effectiveness of treatment (90%, 18/20).
The treatment goal for post-CA SE is seizure suppression or burst suppression for a minimum of 24 hours (95%, 19/20).
Valproic acid and levetiracetam are reasonable first-line agents for seizure treatment after CA (84%, 16/19).
Valproic acid and levetiracetam are reasonable first-line agents for treatment of electroclinical myoclonus or electrographic seizures or SE with electroclinical myoclonus after CA. Clonazepam can be effective, but its sedative effects may confound neurological examination (100%, 20/20).
Do not aggressively treat clinical myoclonus without electrographic correlate unless myoclonic activity interferes with other aspects of care (eg, ventilation) (100%, 24/24).
Do not continue temperature control with a hypothermic target specifically for the treatment of seizures or SE after CA (85%, 17/20).
A full-montage EEG is most sensitive to capture seizures. Limited-montage EEG may be used in select settings (100%, 17/17).
Sedation and Analgesia
Takeaway: Use short-acting sedative and analgesics. Propofol and fentanyl are better options to a benzo or morphine.
Statements:
The goals of analgesia and sedation during temperature control after CA are to provide comfort, to reduce shivering, and to prevent recall during NMB (100%, 21/21).
Short-acting sedative and analgesic agents are preferred for patients in post-CA coma undergoing temperature control to reduce the duration of mechanical ventilation, time to awakening, and confounding of delayed prognostication (100%, 21/21).
Propofol, remifentanil, and fentanyl are favored over midazolam and morphine infusions (85.7%, 18/21).
Use NMB as needed during temperature control rather than as a continuous infusion. In addition, it is important to note that NMB may mask seizures in unmonitored patients (95.3%, 20/21).
Early Triage
Takeaway: Early (< 6 hours) neurologic risk stratification is challenging and inaccurate; avoid early prognostication. However, early risk assessment may help guide clinical interventions.
Statements:
Early risk stratification is not intended as a tool for triage to withdraw life support and is not used for that purpose (90.5%, 19/21).
Data that do not establish neurological risk stratification in the first 6 hours after CA include the patient’s age, duration of CPR, seizure activity, serum lactate level or pH, Glasgow motor subscore in patients who received NMB or sedation, pupillary function in patients who received atropine, and optic nerve sheath diameter (95.3%, 20/21).
Validated illness severity scores may be used to optimize therapeutic interventions (88.2%, 15/17).
Cardiac Management
Takeaway: Perform an echo as soon as you can to guide resuscitation. There are no hard MAP recommendations. Individualize MAP targets based on the patient, as well as treatments (inotropes, vasopressors, and fluids) for hypotensive patients. Evaluate for mechanical support in those with refractory hypotension (ECMO). Authors state early cath may be of benefit in those with no STEMI, but much of the more recent literature suggests this is more controversial. Get your cardiologist on board early.
Statements:
In ICUs where advanced cerebral monitoring is not in routine use, target an MAP >80 mm Hg unless there are clinical concerns or evidence of adverse consequences (82.6%, 19/23).
In patients after CA, perform echocardiography as soon as possible to evaluate right and left ventricular function, cardiac output, and inferior vena cava size to guide hemodynamic management and to search for correctable causes of the CA (95.7%, 22/23).
Serial echocardiography can be helpful to guide ongoing hemodynamic management in patients after CA, at least until unsupported hemodynamic stability occurs (91.3%, 21/23).
The choice of a target post-CA blood pressure incorporates the need to maintain adequate cerebral perfusion during the period of maximal cerebral edema and loss of cerebral autoregulation while accounting for the response of left ventricular function to interventions as assessed by echocardiography (91.3%, 21/23).
Individualize the choice of using inotropes, vasopressors, or fluids to treat post-CA hypotension and to target the likely cause(s) contributing to the shock and hemodynamic state (100%, 23/23).
Serial measurements of central venous oxygen saturation, myocardial oxygen consumption, and lactate are helpful in monitoring the adequacy of systemic perfusion and the effectiveness of therapies used to treat shock (86.4%, 19/22).
In patients with refractory hypoperfusion, evaluate early for mechanical circulatory support (including intra-aortic balloon pump, temporary right or left ventricular assist device, and extracorporeal membrane oxygenation) to improve end-organ perfusion. If mechanical circulatory support is not available, transfer to a center with these capabilities may be possible (95.7%, 22/23).
Early coronary angiography in post-CA patients with no ST-segment elevation on the presenting ECG may still be of benefit by potentially salvaging myocardium and decreasing the incidence of systolic heart failure in survivors (95.7%, 22/23).
Pulmonary Management
Takeaway: Use lung-protective ventilation. Target PaCO2 35-45 mm Hg and saturation 92-98%. You may need to adjust the PaCO2 target to help maintain a pH over 7.2. Blood gas can assist with optimizing ventilation and oxygenation.
Statements:
Lung-protective ventilation is a standard of care for most critically ill patients who are at risk for developing ARDS, including those who remain comatose after CA (92%, 22/24).
Once a reliable arterial oxygen saturation is available after ROSC, titrate Fio2 to achieve an oxygen saturation (Spo2) of 92% to 98% (91.3%, 21/23).
Do not titrate down Fio2 until reliable measurements of the oxygen saturation (Spo2) are available (91.3%, 21/23).
Generally, adjust ventilation to target normal PaCO2 (35–45 mm Hg) after ROSC. There may be specific patients for whom higher or lower CO2 may be appropriate. A higher PaCO2may be appropriate as long as pH can be maintained (>7.2). Alternatively, a slightly lower PaCO2 within the normal range may be used to maintain a safe pH (>7.2) in patients with metabolic acidosis until acidosis can be otherwise treated (94.7%, 18/19).
Hematologic Management
Takeaway: They recommend a transfusion threshold < 9 g/dL in those with ACS, but several studies (MINT trial) and guidelines suggest 8 g/dL can be used. Administer VTE prophylaxis in the first 48 hours, preferably LMWH.
Statements:
As for other critically ill patients, initiate RBCT when hemoglobin is <7 g/dL; however, higher transfusion thresholds (ie, <9 g/dL) may be indicated in patients with acute coronary disease (100%, 21/21).
Individualize RBCTs to the clinical situation (81%, 17/21).
Initiate DVT prophylaxis within 48 hours after admission unless there is contraindication (85.7%, 18/21).
Low-molecular-weight heparin is the first choice for DVT prophylaxis (95.2%, 20/21).
Low-dose heparin, dalteparin, or reduced doses of other low-molecular-weight heparins can be used in patients with kidney dysfunction. Monitoring of anti-Xa activity may be considered when low molecular-weight heparin is used in this setting (90.5%, 19/21).
Digestive Management
Takeaway: Start enteral feeds when the patient gets to the ICU. Start low and go slow. Consider stress ulcer prophylaxis for standard indications, but this may not be necessary if they are receiving feeds.
Statements:
Initiate EN as soon as possible after ICU admission (100%, 20/20).
In patients with enteral intolerance or shock, start with trophic EN (rates of 10–20 mL/h) and adjust according to tolerance (91%, 19/21).
Start parenteral nutrition when enteral feeding is not tolerated or is contraindicated after 5 to 7 days after CA (100%, 21/21).
Give proton pump inhibitor or H2 blockers for stress ulcer prophylaxis per standard indications in the critically ill patient (90.5%, 19/21).
In patients receiving EN, stress ulcer prophylaxis may not be necessary (90.5%, 19/21).
ID Management
Takeaway: Yes, consider antibiotics for patients treated with temperature management. The literature isn’t great, but one dose probably won’t hurt. Don’t use CRP or PCT to guide antibiotics.
Statements:
Empirical antibiotics may be used in patients who are treated with temperature management to a hypothermic target after CA to reduce the incidence of pneumonia (89.5%, 17/19).
Do not use C-reactive protein and procalcitonin to guide antibiotic initiation or duration of therapy (85%, 17/20).
Endocrine and Fluids Management
Takeaway: Fluids should be based on volume status and cause of arrest. If they have increased ICP or cerebral edema, go with NS. Otherwise, administer balanced crystalloids. Sodium bicarbonate should not be used routinely, but consider in patients with AKI and pH < 7.2.
Statements:
Volume management takes into consideration the cause of arrest, hemodynamic target chosen, and underlying organ dysfunction and is individualized to each patient (100%, 23/23).
Balance the risk for cerebral edema with complications associated with hyperchloremia when choosing intravenous fluid after CA. The preferred choice in the setting of cerebral edema is normal saline, although balanced crystalloid solutions may minimize hyperchloremia and the potential for AKI when cerebral edema is not present (95.7%, 22/23).
Do not use sodium bicarbonate routinely in patients after CA who have metabolic acidosis. Sodium bicarbonate may be considered in patients with severe metabolic acidosis (pH <7.2, bicarbonate <20) and AKI stage 2 or 3 (82.6%, 19/23).
Consider RRT after CA for when life-threatening changes in fluid, electrolytes, and/or acid-base balance exist and for conditions that can potentially be modified with RRT (100%, 23/23).
Do not administer empirical corticosteroids to all patients after CA, although supplemental corticosteroids may be useful to treat persistent shock in patients with proven or suspected adrenal suppression (91.3%, 21/23).
Consider treatment of hyperglycemia with glucose targets of 81 to 180 mg/dL (100%, 19/19).
Treatment Protocols, Family Support, and Team-Based Care Statements
Takeaway: Protocols for management are essential to ensure we optimize and improve our care of post arrest patients. Multidisciplinary is best if possible.
Statements:
Establish structured treatment protocols with input from multiple disciplines, including emergency, cardiology, critical care, neurology, nursing, and pharmacy, for an integrated multidisciplinary approach (91.3%, 21/23).
Centers managing patients after CA should collect data and evaluate outcomes of care (95.7%, 22/23).
Centers caring for patients after CA, particularly patients with neurological deficits, should offer specialized post-CA care, including the following: Cardiac catheterization team available 24 h/d and 7 d/wk, Temperature control available 24 h/d and 7 d/wk, Diagnostic testing available for prognostication, Intensive care team with specialty knowledge in post-CA care, Practitioners with expertise in interpretation of diagnostic testing for prognostication after CA, Patient and family support at discharge and provision of follow-up care after CA, and EEG monitoring capabilities (82.6%, 19/23).
In patients after CA who remain unresponsive after ROSC, structured treatment protocols can be helpful and defined in terms of bundles of care with a specific goal-directed approach (85.7%, 18/21).
Goals-of-Care Discussions and Family/Surrogate Support Statements
Takeaway: Include surrogate decision makers, and we need to know how to perform a goals-of-care discussion.
Statements:
Include patients and/or surrogate decision makers as active participants in care and care decisions. These conversations should occur frequently and be clearly documented for other health care professionals who are not present during the conversations (96%, 22/23).
Clinicians involved in goals-of-care decision-making may benefit from additional training to develop key skills and to address individual biases when facilitating these discussions (96%, 22/23).
Neurological Assessment Statements
Takeaway: Perform repeat neurologic exams.
Statements:
Assess neurological status frequently in patients who have an abnormal neurological examination after CA (90%, 18/20).
Patients who are in a coma may benefit from a comprehensive neurological assessment. The below table gives the components of the neurological examination when a patient is unresponsive (100%, 20/20).
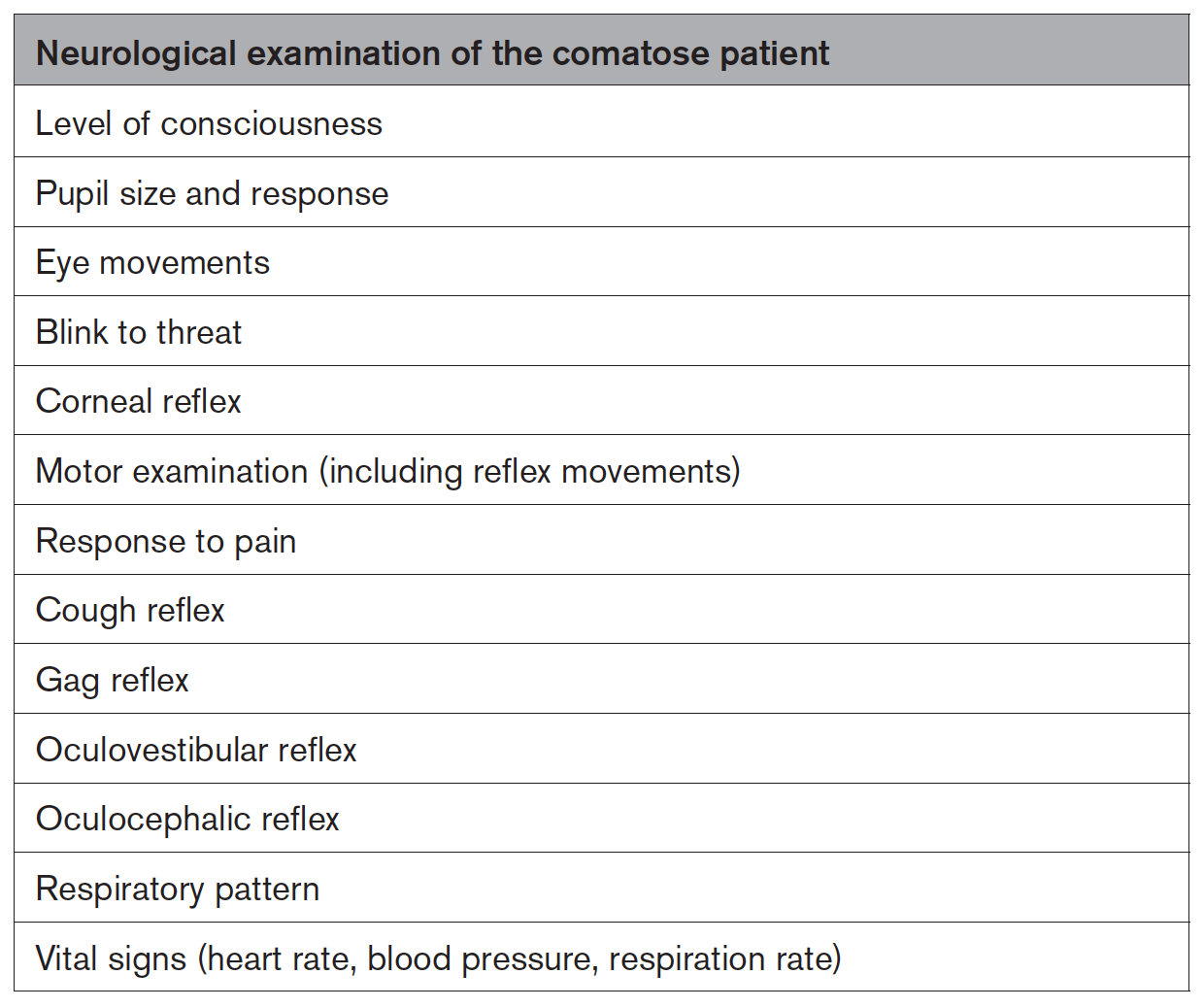
Evaluate quantitative pupillometry in patients who have an abnormal neurological examination and/or who are receiving significant doses of sedatives, analgesics, or paralytics (95%, 19/20).
Changes in a neurological examination prompt timely evaluation of the need for further diagnostic tests to identify potentially treatable causes. The clinical treatment team has the ability to respond to monitoring changes by optimizing postresuscitation care (100%, 18/18).
Reference: