Author: Chris Chase, MD (EM Resident Physician, Parkland Memorial Hospital / UT Southwestern Medical Center) // Edited by: Alex Koyfman, MD (@EMHighAK, EM Attending Physician, UT Southwestern Medical Center / Parkland Memorial Hospital) and Brit Long, MD (@long_brit)
During your busy Monday afternoon shift you go to evaluate what seems to be your 10th abdominal pain complaint of the day. This patient is a 20 year-old female that presents with two days of periumbilical abdominal pain, which has migrated to her RLQ and is associated with N/V. LMP was 3 weeks ago. She denies urinary symptoms, vaginal bleeding/discharge, and diarrhea. Her vitals are BP 118/79, pulse 105, RR 21, O2 sat 99%, and T 101.2. On exam she is tender in her RLQ and without CVAT. As you are walking out the room thinking you have already made the diagnosis of appendicitis, she mentions that this pain feels very similar to when she needed an appendectomy three years ago. What other conditions mimic appendicitis that you need to consider?
Appendicitis Background:
Abdominal pain is a high volume complaint in the ED, and there are approximately 250,000 cases of appendicitis each year. This is a high-risk complaint: 10% of total malpractice suits against EPs involve a missed diagnosis of abdominal pain [1]. Appendicitis carries a lifetime risk of 7-8% for all patients, with 70% of cases occurring in patients less than 30 years of age and more commonly in males [2]. Appendicitis has a complex range of symptoms and atypical presentations, which can lead to missed diagnosis and potential malpractice claims. Alternatively, other etiologies may mimic appendicitis’ presentation and lead to unnecessary appendectomies, more commonly in females due to gender-specific pelvic etiologies [1].
Appendicitis classically presents with RLQ tenderness, migration of pain from periumbilical to RLQ, and pain prior to vomiting over the course of 12-24 hours. There is no individual symptom or physical exam finding that can reliably exclude appendicitis, and atypical presentations are very common [2]. Atypical presentations are common in obese, extremes of age, and diabetics [3]. Workups include CBC, urinalysis, and urine pregnancy testing in women, but the diagnosis is commonly a clinical diagnosis. Elevated C reactive protein (CRP) has been shown to aid in the detection of abscess formation, with 90% of complicated appendicitis having a CRP greater than 99mg/L [4]. Pelvic exam should be considered in female patients with undifferentiated lower abdominal pain; however, cervical motion tenderness does not rule out appendicitis [3].
There are three major scoring systems used to risk stratify patients with suspected appendicitis: Alvarado Score (AS), the Pediatric Appendicitis Score (PAS), and the Appendicitis Inflammatory Response Score (AIRS). These scoring systems help determine who may need further imaging studies, but they do not determine the need for surgical intervention alone [2].
ALVARADO score: (MANTRELS)
Migration of pain RLQ (1 point)
Anorexia (1 point)
N/V (1 point)
Tenderness in RLQ (2 point)
Rebound pain (1 point)
Elevated temp >= 37.3 (1 point)
Leukocytosis >=10 (2 point)
Shift of WBC to left (1 point)
Score of 7 or more has positive LR of 4.0. Score of less than 7 has negative LR of 0.2 [3].
The American College of Emergency Physicians Clinical Policy (2010) recommend risk stratification and evaluation with abdominal CT (with or without IV/PO contrast) in adults with suspected appendicitis. In pediatric populations, US is used to diagnose, but not exclude appendicitis. Pregnant women should be evaluated by ultrasound followed by MRI, if needed [2]. ED management of suspected/confirmed appendicitis includes pain management, IV fluids, antibiotics, and surgical consultation. Antibiotics with aerobic and anaerobic coverage should be used.
Further reading regarding evaluation, diagnosis and management can be found at: http://www.emdocs.net/appendicitis-pearls-and-pitfalls-in-adult-and-pediatric-populations/.
As emergency physicians, we commonly are fixed on ruling out appendicitis in a patient presenting with RLQ abdominal pain but don’t always consider etiologies that may be mimicking its presentation. We will discuss the etiologies of right lower quadrant pain that are essential for the emergency physician to consider that are often indistinguishable from the atypical presentations of appendicitis. These include ectopic pregnancy, ovarian/testicular torsion, pelvic inflammatory disease/TOA, terminal ileitis, cecal diverticulitis, cecal volvulus, gastroduodenal perforation, intussusception, Crohn’s Disease, ureterolithiasis, biliary colic, epiploic appendagitis, omental infarction, and mesenteric adenitis.
Mimics
Ectopic Pregnancy:
An ectopic pregnancy occurs when a fertilized ovum implants outside the uterine cavity, with 98% implanting in the fallopian tube [5]. Ectopic pregnancy has high morbidity and mortality associated with it, secondary to tubal rupture and life-threatening hemorrhage, and must be considered in female patients presenting with lower abdominal pain [6]. Patients commonly present between 6-10 weeks gestation with lower unilateral abdominal pain and vaginal bleeding; however, 9% have been found to have no pain. Cervical motion tenderness has been reported present in 67% of cases. Risk factors for ectopic include PID, previous pelvic/abdominal surgery, smoking history, advanced maternal age, previous ectopic and IVF treatment. A ruptured ectopic pregnancy should strongly be suspected in a woman that presents with a positive pregnancy test and signs of shock including tachycardia, pallor or syncope [5]. Diagnosis should be made by transvaginal ultrasound (TVUS) and serum beta hCG. If the serum hCG is above the discriminatory zone (2000 international units/L), then a gestational sac should be visualized by TVUS if an IUP is present [6]. If no IUP is identified than serial hCG measurements in 48 hours (normal levels increase by 66%) are used to determine intrauterine viability. The identification of a non-cystic adnexal mass with an empty uterus by TVUS has a sensitivity of 84%-90% and a specificity of 94-99% for ectopic gestation. Suspicion increases if free fluid is visualized [5]. Heterotopic pregnancy (combined intrauterine and extrauterine pregnancy) should be considered in women with symptoms who have undergone in vitro fertilization [6].
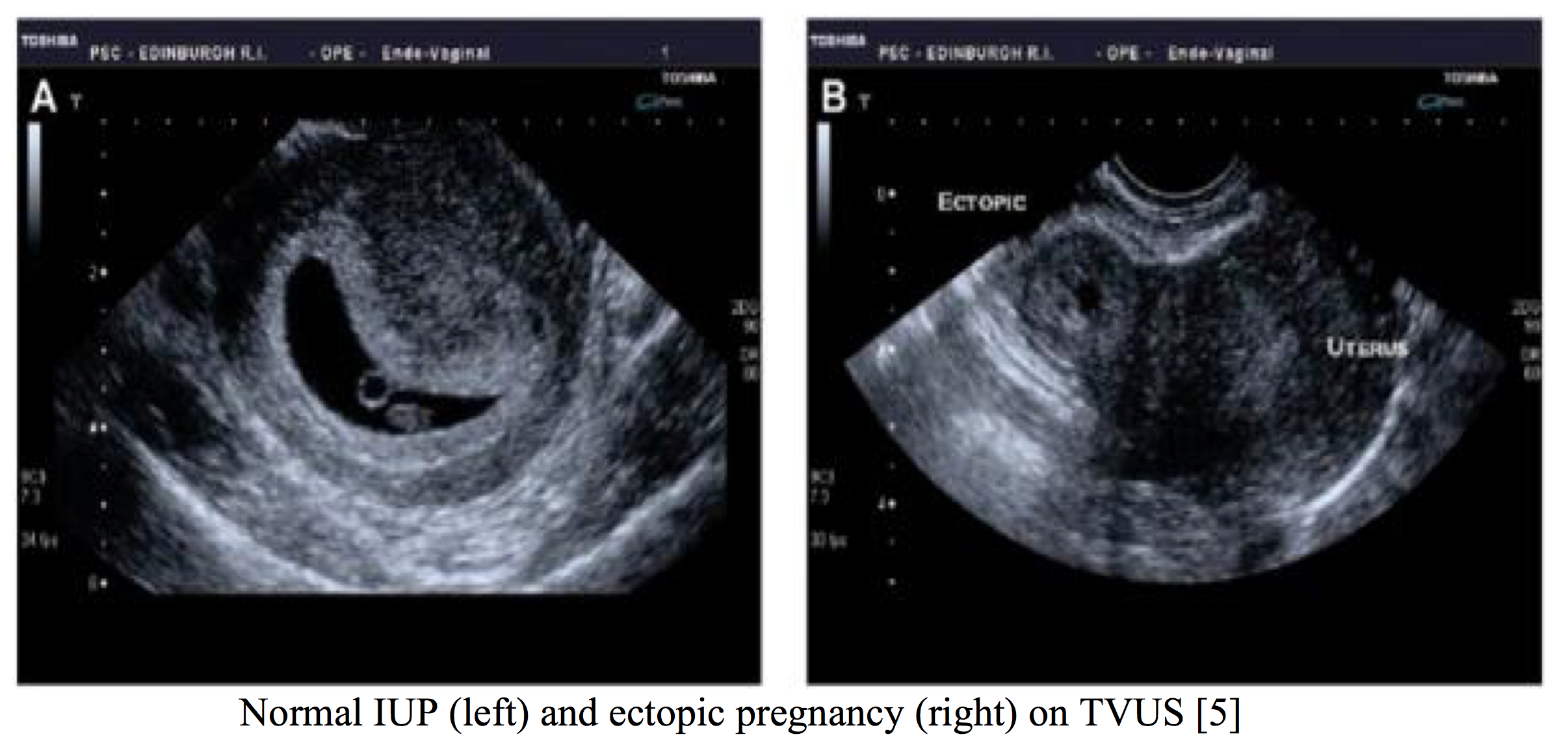
Ectopic pregnancy may be managed by immediate surgical intervention, medically with methotrexate or expectant management depending on presentation and severity of condition. If an ectopic pregnancy has ruptured, laparoscopy is required. Methotrexate can be given to unruptured tubal pregnancies in women whom are hemodynamically stable, with minimal symptoms and a low volume of free fluid. Expectant management may be considered if an ectopic is resolving spontaneously by regression or tubal abortion [5].
Ovarian/Testicular Torsion:
Ovarian torsion is a gynecological emergency with approximately 4.9 cases per 100,000 a year. Torsion is caused by the ovary rotating around its supporting ligaments, leading to occlusion of lymphatics and vasculature [7]. Ovarian torsion can often be mistaken for appendicitis as it is more common on the right, presents with sudden onset unilateral abdominal pain, and vomiting is seen in 60% of cases. Pain can be intermittent due to detorsion [8]. Risk of torsion increases with ovarian mass (4-5 cm), but there is no obvious ovarian pathology in 58% of cases. Children comprise 15% of cases with the highest rates occurring in the 9-14 age range [7]. Diagnosis is made by ultrasound with Doppler flow (transabdominal in children, transvaginal in older females). The most common sign on ultrasound is an enlarged ovary. Blood supply to the ovary includes the tubal and ovarian branches of the uterine artery, and due to the two blood supplies, the US may show normal arterial flow [7]. Decreased or absent flow in a patient with pelvic pain and an adnexal mass is 100% sensitive and 97% specific for torsion [8]. Definitive management with surgical correction is needed to reverse the torsion and preserve ovarian function. Management with IVF and broad-spectrum antibiotics should be initiated if signs of sepsis or peritonitis are present [8].
Testicular torsion is a urologic emergency, occurring in 1 in 4000 males younger than 25 years of age and a peak incidence during puberty (ages 12-15) [10]. Torsion is classically caused by a “bell-clapper deformity” where there is a high attachment of the tunica vaginalis, allowing the testicle to rotate around the spermatic cord. This rotation leads to venous congestion, arterial compromise, and ischemia of the testicle. Torsion typically presents as sudden, severe unilateral testicular pain associated with nausea and vomiting; however, 20-30% of cases present as lower abdominal pain [9]. This highlights the necessity for testicular exam in a young male complaining of lower abdominal pain. Leukocytosis and pyuria can be seen in approximately 30% of cases, often mimicking epididymitis [9]. A horizontal, high-riding testicle and absent cremasteric reflex implies torsion. Presence of a cremasteric reflex does not adequately rule out torsion. [10]. Testicular ultrasound should not delay the diagnosis, and spontaneous detorsion may yield normal Doppler flow to the testicle. Surgery is the only definitive treatment, with a salvage rate of 96% if perfusion is restored in less than 4 hours. Salvage rates fall to less than 10% if intervention is delayed for more than 24 hours. Manual detorsion (rotating the testicle in the “open book” direction with a goal of 540 degrees) may be attempted if surgical treatment is delayed [10].
Pelvic Inflammatory Disease/TOA:
Pelvic Inflammatory Disease (PID) is an infectious process of the upper genital tract and comprises the most ED gynecological visits. The most common pathogens are Neisseria gonorrhoeae and Chlamydia trachomatis, but 20% of cases do not have an inciting pathogen. Ninety percent of patients present with bilateral lower abdominal pain, with 75% of cases occurring within the first seven days of menses. New vaginal discharge is not specific or sensitive. Approximately half of patients have fever [11]. The most useful component is the pelvic exam with mucopurulent endocervical discharge, cervical motion tenderness, and bilateral adnexal tenderness highly suggestive of PID. Risk factors include prior PID, high number of sexual partners, and unprotected sex [12]. There should be a low threshold for empiric treatment for presumed PID. Complications of PID include increased risk for ectopic pregnancy, infertility, and chronic pelvic pain. Most cases are treated as an outpatient, whereas only 10-25% are hospitalized [11]. The CDC recommends a fourteen-day course of antibiotics. The most common antibiotic regimen is Ceftriaxone 250 mg IM once plus doxycycline 100 mg BID x 14 days. Further information regarding antibiotic therapy options for PID can be found at: https://www.cdc.gov/std/tg2015/pid.htm. [13]
The most immediate life-threatening complications of PID is tubo-ovarian abscess (TOA). This occurs in approximately one-third of patients with diagnosed PID when purulence is spread to the ovary via the fallopian tube. TOA is an emergent polymicrobial infection. Diagnosis is made by TVUS with a sensitivity of 93% and specificity of 98% in patients with clinically diagnosed PID [11]. Abscess rupture is a surgical emergency, leading to sepsis and ultimately death if not treated. Patients must be hospitalized with aggressive resuscitation, broad-spectrum IV antibiotics, and surgical evaluation if abscess rupture is suspected.
Right-Sided Diverticulitis:
Cecal diverticulitis is a rare disease in Western countries, with an incidence of 1.5% of patients presenting with diverticulitis. Right-sided diverticula occur more often as true diverticula, with younger populations. Asian countries have a higher rate of cecal diverticulitis (75% of presenting diverticulitis cases) [14]. Symptoms typically mimic appendicitis with right lower quadrant pain, nausea, vomiting, low-grade fever, and leukocytosis. Due to this presentation, diagnosis of cecal diverticulitis is commonly made in the operating room. It was found that 70% of patients found to have cecal diverticulitis were operated on with preoperative diagnosis of acute appendicitis and was found in 1 in 300 appendectomies [15]. Diagnosis by CT has a high sensitivity and specificity (98%) and may prevent unnecessary surgical exploration. Treatment is controversial for non-perforated cecal diverticulitis. Preoperative diagnosis is treated with bowel rest and IV antibiotics. If diagnosed intraoperative, appendectomy and possible diverticulectomy is performed; however, if large amounts of inflammation or abscess is found then right hemicolectomy is recommended by most surgeons to prevent recurrence [16].

Terminal Ileitis/Enteritis:
Terminal ileitis is often caused by Crohn’s Disease, but many infectious etiologies may cause similar presentations that are often indistinguishable from acute appendicitis. Patients may present with acute right lower quadrant pain, fever, leukocytosis, and mild/bloody diarrhea. Most cases are self-limiting, but some may lead to obstruction or hemorrhage [17]. Yersinia enterocolitica can cause enterocolitis after ingesting contaminated food or water and may present as pseudoappendicitis. Patients complain of right lower quadrant pain, and 15-40% of cases have vomiting [17]. Bloody diarrhea has been seen in 20-60% of cases, and pharyngitis may be a presenting symptom in 20% of cases (helpful in distinguishing from other causes of ileitis) [18]. Definitive diagnosis is made by stool culture, and CT reveals a thickened and nodular mucosal pattern in the terminal ileum. Yersinia can also lead to mesenteric adenitis with terminal ileitis, mimicking appendicitis [17]. Campylobacter colitis is a common bacterial foodborne illness affecting 2.4 million people a year. Patients may present with fever, abdominal pain, and bloody stools, often requiring fourteen-day courses of antibiotics. Diagnosis is made with stool culture, and most self-limiting infections are treated with supportive care [19]. Salmonella is one of the most common foodborne illnesses in the U.S. caused by ingesting contaminated animal food products. Salmonella is typically a self–limiting gastroenteritis but can lead to septicemia in pediatric, immunocompromised, and geriatric patients [17]. Neutropenic or immunocompromised patients can present with typhlitis, an acute life-threatening inflammatory condition of the cecum and ascending colon that can progress to transmural necrosis and perforation [20]. Enterohemorrhagic Escherichia coli (EHEC) produces an enterotoxic Shiga toxin (similar to Shigella), leading to abdominal cramping, tenesmus, and bloody/mucoid stools. Treatment is primarily supportive, but anti-motility and antibiotics are contraindicated in these cases, as they may lead to increased toxin release. Hemolytic uremia syndrome (HUS) is a major complication (6-9%) of EHEC infections that occurs 5-10 days after diarrhea with renal failure, hemolytic anemia, and thrombocytopenia. [21].
Crohn’s Disease:
The initial presentation of Crohn’s Disease (CD) can be similar to acute appendicitis. Patients may present with abdominal pain, anorexia, fever, weight loss, and diarrhea with hematochezia. Symptoms typically occur in a relapsing and remitting nature with intermittent symptoms. The exact etiology of Crohn’s is unknown; however, genetic influences, diet, and cigarette smoking may play a role. Annually in the U.S. and Canada, 10,000-47,000 people are diagnosed with CD. Diagnosis is most commonly made in late adolescence and early adulthood with a mean age of 33 [22]. Crohn’s is a transmural inflammation of the bowel wall anywhere from the mouth to anus [23]. It is imperative for the emergency physician to identify complications associated with CD that may lead to acute abdomen, sepsis, and GI hemorrhage. CD has a propensity to involve the terminal ileum and appendix causing RLQ abdominal tenderness. The most common site affected by CD was found to be ileocolic (41%) [24]. Labs are non-specific but may show microcytic/normocytic anemia, leukocytosis, thrombocytosis, elevated CRP/ESR, and mild elevations in LFTs. CT with IV/PO contrast is used to rule out complications such as abscess, bowel perforation, and obstruction in the acute phase. Treatment in acute flares is with IV fluids, IV steroids, and antibiotics (ciprofloxacin and metronidazole) if infectious colitis is suspected. Broad-spectrum antibiotics should be given and surgical consult obtained if abscess, perforation, or toxic megacolon is found. Toxic megacolon is a surgical emergency, seen in 1% of patients with inflammatory bowel disease, which can lead to perforation, sepsis, and hemorrhage [25].
Cecal Volvulus:
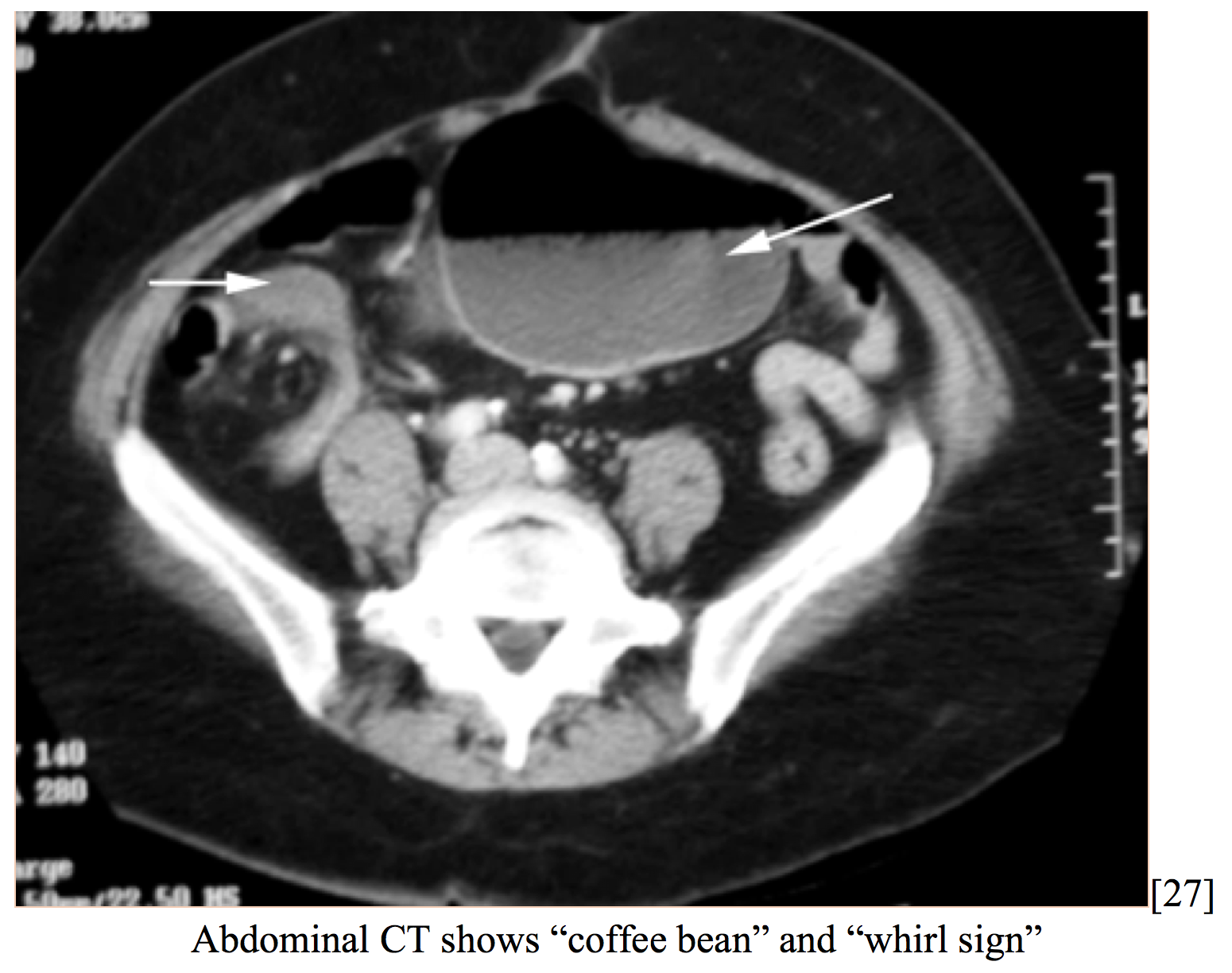
Cecal volvulus is a rare cause of intestinal obstruction (1-1.5% of all obstructions), caused by axial twisting of the cecum along the terminal ileum and ascending colon. Patients may present with abdominal pain, distension, nausea, vomiting, and diarrhea or constipation. There is thought to be an anatomical predisposition due to incomplete intestinal rotation leading to inadequate right colon fixation [26]. 23-53% of patients presenting with cecal volvulus had prior abdominal surgery. Patients already hospitalized are at an increased risk (12-28% of cases), due to dysmotility [27]. Volvulus is rarely diagnosed correctly at time of presentation due to low incidence. CT reveals cecal distension, cecal apex in left upper quadrant, small bowel distension, mesenteric whirl, and absence of gas in colon [26].
Patients frequently have a history of mobile caecum syndrome (50% of cases presenting with acute volvulus), characterized by recurrent, intermittent RLQ pain and distension that resolves with passing flatus. Acute volvulus presents very similar to SBO and can lead to peritonitis, gangrene and hemodynamic compromise [27]. Early laparoscopic surgical intervention by untwisting the cecum and performing cecopexy is the definitive treatment. Perioperative mortality ranges from 0-40% depending on bowel viability and need for gangrenous resection [26].
Intussusception:
Intussusception is a rare but important diagnosis to consider in adult populations. Whereas 90% of pediatric cases are idiopathic, structural lesions cause the majority of cases found in adults. Often, malignant neoplasms (adenocarcinoma most commonly) are found. Intussusception is caused by one segment of bowel “telescoping” into an adjacent segment leading to obstruction, inflammation, and ischemia. This is the leading cause of obstruction in children but accounts for less than 5% of bowel obstructions in adults. The classic presentation in pediatrics (15% of cases) of abdominal pain, vomiting, and currant-jelly stools are infrequently found in adults [28]. In adults, the most common symptom is abdominal pain (70-80%), but 20% may be asymptomatic [29]. Pediatric populations also have non-specific symptoms, such as paroxysmal abdominal pain, vomiting, bloody stools, and a palpable abdominal mass in the RUQ. Diagnosis is often initially misdiagnosed (60% cases) as viral gastroenteritis. Pediatric intussusception is often preceded by viral infection and can be seen as a complication of other disease processes like CF and HSP [30]. Adult intussusception involves the small bowel in 52% of cases (39% enteroenteric, 13% ileocolic), whereas pediatric cases are 90% ileocolic [28]. Ultrasound should be used to diagnose pediatric cases, illustrating a “target” or “doughnut” appearance. If US is unavailable or questionable then air enema may be used for both diagnosis and treatment. Treatment with air-contrast enema is used with a 60-90% reduction rate in children [30]. CT scan is the preferred imaging for adults, which usually shows the lead point. Adults are managed surgically, as the underlying etiology is structural [31].
Gastroduodenal Perforation:
Gastroduodenal perforation is an emergent diagnosis that requires a high index of suspicion and carries a high morbidity and mortality (10-40%). PUD affects approximately 4 million people worldwide, with 2-14% of ulcers perforating [32]. Patients classically present with acute onset epigastric abdominal pain that generalizes to lower quadrants with peritoneal signs; however, peritonitis may be minimal in patients with contained leaks. Helicobacter pylori is implicated in 70-90% of all perforated duodenal ulcers, and 40-50% of cases have NSAIDs playing a causative role. Labs are often non-specific acutely but may reveal metabolic acidosis and leukocytosis. Fever and hypotension is typically a late finding.

Upright CXR reveals free air in 80% of cases [33]. CT abdomen with IV and PO contrast can further characterize intra-peritoneal fluid and pneumoperitoneum and improve diagnostic accuracy [32]. In 40-50% of cases, the ulcer self-seals with omentum. Patients should be resuscitated, nasogastric suction begun, and broad-spectrum antibiotics administered immediately. If ongoing signs of peritonitis or pneumoperitoneum are present, then operative laparoscopy treatment with omental patch, abdominal lavage, and subsequent eradication of H. pylori is recommended [33].
Ureterolithiasis:
Kidney stones are very common and seen in 16 of 10,000 patients. Patients present with acute onset, colicky severe flank pain radiating to lower abdomen associated with nausea and vomiting (20%). Patients often have hematuria; however, absence of hematuria does not rule out a stone [35]. When a stone is obstructed in the ureterovesical junction a patient may also present with dysuria and frequency. Calculi are caused by super-saturation of a urinary solute causing crystal formation. Most stones are made of calcium combined with oxalate and/or phosphate and comprise 80% of all stones [36]. Uric acid, struvite, and cysteine stones account for the remainder of stones in decreasing order. Patients typically have high recurrence rates. Risk factors include high diet intake of salt, calcium, purine, and proteins, as well as some medications such as diuretics, antacids, and allopurinol [35]. Non-contrast CT is used to diagnose stones with a sensitivity of 97% and specificity of 96%, but renal US can also be used [36]. Treatment is focused on adequate hydration, pain control, and ruling out concomitant infection. Stones smaller than 4 mm pass spontaneously 80% of the time, and 60% pass if 4-6 mm in size. Patients should be admitted for large stones unlikely to pass, uncontrolled pain or vomiting, infection, pregnancy, or persistent obstruction [35].
UTI/Pyelonephritis:
There are 6-7 million episodes of acute cystitis and 250,000 episodes of pyelonephritis each year in the U.S., with a majority of cases in young sexually active females. Pyelonephritis is an infection of the renal parenchyma and pelvis from local bacterial infection. E coli is the most common pathogen, causing 70-95% of UTIs and 80-90% of pyelonephritis cases. Other causative bacteria include Staphylococcus saprophyticus, Proteus, Klebsiella, Enterococcus, and Pseudomonas. Sexual intercourse, urinary obstruction, bladder dysfunction, and vesicoureteral reflux increases risk [35]. Patients with acute cystitis present with dysuria, urgency, frequency, and suprapubic abdominal pain. Pyelonephritis presents with flank pain, CVA tenderness, nausea, vomiting, fever, and leukocytosis [37]. Many patients with pyelonephritis report cystitis symptoms but 50% of cases present without them. Urinalysis reveals WBCs, bacteriuria, and nitrites. Leukocyte esterase in the urine is 75-90% sensitive and 94-98% specific for UTI. Hematuria is found in 40% of cases [35]. Sterile pyuria can be a sign of intra-abdominal infection, such as with acute appendicitis, due to irritation of the bladder or ureter, which results in increased urinary RBC/WBC counts [38]. Imaging with renal ultrasound or CT is indicated in patients with complicated pyelonephritis to assess for hydronephrosis or abscess. Pregnant or immunocompromised patients and patients with known obstruction or renal impairment with pyelonephritis are considered complicated and should be admitted for IV antibiotics [39]. Treatment of pyelonephritis is most commonly with outpatient fluoroquinolones for 10-14 days. If local fluoroquinolone resistance prevalence of E. coli exceeds 10%, an initial intravenous dose of ceftriaxone or gentamicin is recommended, followed by an oral fluoroquinolone regimen [37].
Biliary Colic/Cholecystitis:
Gallbladder disease should be considered in patients that present with right-sided abdominal pain. Gallstones are a very common disorder, with an estimated 20.5 million cases of gallbladder disease in the U.S. Over 600,000 cholecystectomies are performed each year in the U.S. due to symptomatic gallstone disease. Risk factors for gallstones include female sex, DM, rapid weight loss, obesity, cirrhosis, and TPN administration [40]. Patients often have intermittent right upper quadrant or epigastric pain (<3 hours with colic) associated with nausea and vomiting. If cystic duct obstruction persists, the gallbladder becomes acutely inflamed and infected, causing cholecystitis. Patients may have fever, leukocytosis, hyperbilirubinemia, and elevated LFTs [41]. Diagnosis is made by RUQ ultrasound showing gallstones, thickened gallbladder wall, and pericholecystic fluid [40]. Treatment for symptomatic gallstones and cholecystitis is laparoscopic cholecystectomy. Patients may also have extrahepatic biliary obstruction in the common bile or common hepatic duct leading to choledocholithiasis. Ten percent of patients with gallstones have common bile duct stones. Patients can present with upper abdominal pain, nausea, vomiting, fever, and jaundice. Biliary obstruction and cholestasis can lead to infection of the bile duct (bacterial cholangitis) or gallstone pancreatitis. Endoscopic retrograde cholangiopancreatography (ERCP) and IV antibiotics are recommended if biliary obstruction is suspected [40].
Epiploic Appendagitis:
Epiploic appendagitis is an uncommon diagnosis caused by torsion of small pouches of peritoneum (filled with fat and small vessels) protruding from serosal surfaces of the colon [42]. Patients present with localized abdominal pain and to a lesser degree nausea, vomiting, leukocytosis, and anorexia. Epiploic appendagitis commonly occurs at the rectosigmoid junction, but is also seen at the ileocecal junction [20]. Symptoms frequently last less than a week and are self-limiting. Treatment is supportive with analgesics.
Omental Torsion/Infarction:
Omental infarction is caused by torsion of fatty tissue of the omentum presenting with acute onset of abdominal pain, often indistinguishable from appendicitis [43]. Idiopathic infarction is commonly precipitated by coughing, straining, or overeating. CT shows a well-circumscribed inflamed fatty mass [42]. Omental infarction is a self-limiting cause of acute RLQ abdominal pain and is treated with analgesics.
Mesenteric Adenitis:
Mesenteric adenitis is a self-limiting inflammation of mesenteric lymph nodes, commonly without an identifiable or underlying cause [42]. It is reported as the second most common cause and the discharge diagnosis of 2-14% of patients presenting with right lower quadrant pain. Mesenteric adenitis is more common in pediatric populations. Patients with appendicitis commonly have mesenteric adenitis concomitantly, so a normal appendix must be visualized to rule out appendicitis. Treatment for idiopathic mesenteric adenitis is with anti-inflammatories, and most cases resolve spontaneously [44].
Summary:
- Abdominal pain is a common and high-risk ED complaint. Appendicitis and its mimics are often indistinguishable in their presentation.
- Appendicitis mimics requiring consideration include: ectopic pregnancy, ovarian/testicular torsion, pelvic inflammatory disease/TOA, terminal ileitis, cecal diverticulitis, cecal volvulus, gastroduodenal perforation, intussusception, Crohn’s Disease, ureterolithiasis, cholecystitis, etc.
- Every reproductive-aged female should have a pregnancy test, and males should have testicular exam when presenting with lower abdominal pain.
- Epiploic appendagitis, omental infarction, and mesenteric adenitis seen on CT are typically benign, self-limited disease processes.
- Early surgical consultation required if suspecting: ruptured ectopic, testicular/ovarian torsion, ruptured TOA, cecal volvulus, gastroduodenal perforation, adult intussusception, cholecystitis, or toxic megacolon.
References/Further Reading:
- Cole MA, M. N. (2011). Evidence-based management of suspected appendicitis in the emergency department. Emergency Medicine Practice, 13(10), 1-32. Retrieved from ebmedicine.net
- Zeretzke-Bien, Cristina M., and Carolyn Holland. “Appendicitis: Pearls and Pitfalls in Adult and Pediatric Populations.” EmDOCs.net – Emergency Medicine Education. N.p., 21 Apr. 2017. Web. 08 May 2017
- Helman, Anton, Claire Heslop, and Brian Steinhart. “Episode 43 – Appendicitis Controversies.” Audio blog post. N.p., 22 Apr. 2014. Web
- Sammalkorpi HE, L. A. (2015). High admission C-reactive protein level and longer in-hospital delay to surgery are associated with increased risk of complicated appendicitis. Langerbecks Archives of Surgery, 400, 221-28
- Sivalingam, Vanitha N et al. “Diagnosis and Management of Ectopic Pregnancy.” The journal of family planning and reproductive health care / Faculty of Family Planning & Reproductive Health Care, Royal College of Obstetricians & Gynaecologists 37.4 (2011): 231–240. PMC. Web. 8 May 2017.
- Tulandi, Togas. “Ectopic Pregnancy: Clinical Manifestations and Diagnosis.” UpToDate. N.p., 2 May 2017. Web. 11 May 2017.
- Fox, Sean. “Ovarian Torsion.” Pediatric EM Morsels. N.p., 24 July 2015. Web. 08 May 2017
- Streitz, Matthew. “Ovarian Torsion: Pearls and Pitfalls.” EmDOCs.net – Emergency Medicine Education. N.p., 21 Apr. 2017. Web. 08 May 2017.
- Hals, Gary D., Tom Dietrich, and David Ford. “Diagnosis and Emergency Department management of urologic emergencies in the male patient.” Emerg Med Rep 23.2 (2002): 17-28.
- Thomas, Vladimir, and James Willis. “Testicular Torsion: Pearls and Pitfalls.” EmDOCs.net – Emergency Medicine Education. N.p., 21 Apr. 2017. Web. 08 May 2017.
- Williamson, Kelly, and Amer Z. Aldeen. “Focus On: Emergent Evaluation and Management of Pelvic Inflammatory Disease.” American College of Emergency Physicians, Jan. 2010. Web. 08 May 2017
- Kruszka, Paul S. Kruszka|Stephen J. “Evaluation of Acute Pelvic Pain in Women.” Evaluation of Acute Pelvic Pain in Women – American Family Physician. N.p., 15 July 2010. Web. 08 May 2017.
- “Sexually Transmitted Diseases Treatment Guidelines, 2015.” Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 05 June 2015. Web. 11 May 2017.
- Dana A. Telem, Kerri E. Buch, Scott Q. Nguyen, Edward H. Chin, Kaare J. Weber, and Celia M. Divino, “Current Recommendations on Diagnosis and Management of Right-Sided Diverticulitis,” Gastroenterology Research and Practice, vol. 2009, Article ID 359485, 4 pages, 2009. doi:10.1155/2009/359485
- Karatepe, Oguzhan et al. “Cecal Diverticulitis Mimicking Acute Appendicitis: A Report of 4 Cases.” World journal of emergency surgery : WJES 3 (2008): 16. PMC. Web. 8 May 2017.
- Nikolaos Mudatsakis, Marinos Nikolaou, Konstantinos Krithinakis, Michail Matalliotakis, Nikolaos Politis, and Emmanouil Andreadakis, “Solitary Cecal Diverticulitis: An Unusual Cause of Acute Right Iliac Fossa Pain—A Case Report and Review of the Literature,” Case Reports in Surgery, vol. 2014, Article ID 131452, 5 pages, 2014. doi:10.1155/2014/131452
- DiLauro, Steven, and Nancy F. Crum-Cianflone. “Ileitis: When It Is Not Crohn’s Disease.” Current gastroenterology reports 12.4 (2010): 249–258. PMC. Web. 8 May 2017.
- Tauxe, Robert V. “Clinical Manifestations and Diagnosis of Yersinia Infections.” UpToDate. N.p., 29 Jan. 2016. Web. 12 May 2017.
- Zhou Eric P., Goldberg Daniel J., Jung Eric E., Dunican Annmarie, and Heffernan Daithi S. Surgical Infections Case Reports. February 2017, 2(1): 20-22. doi:10.1089/crsi.2017.0001
- Thompson JP, Selvaraj D, Nicola R. Mimickers of Acute Appendicitis. J Am Osteopath Coll Radiol. 2014;3(4):10-21.
- Calderwood, Stephen B. “Clinical Manifestations, Diagnosis and Treatment of Enterohemorrhagic Escherichia Coli (EHEC) Infection.” UpToDate. N.p., May 2016. Web. 12 May 2017.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence and environmental influences. Gastroenterology 2004;126(6):1504-17.
- Shaoul, Ron et al. “Crohn’s Disease and Recurrent Appendicitis: A Case Report.” World Journal of Gastroenterology 11.43 (2005): 6891–6893. PMC. Web. 8 May 2017.
- Farmer, R. G., W. A. Hawk, and J. R. Turnbull. “Clinical Patterns in Crohn’s Disease: A Statistical Study of 615 Cases.” Gastroenterology. U.S. National Library of Medicine, Apr. 1975. Web. 08 May 2017.
- Trueblood, Wes, and Erica Simon. “Inflammatory Bowel Disease in the Emergency Department.” EmDOCs.net – Emergency Medicine Education. N.p., 25 Oct. 2016. Web. 8 May 2017.
- Consorti ET, Liu TH Diagnosis and treatment of caecal volvulus Postgraduate Medical Journal 2005;81:772-776.
- Atamanalp, S. Selcuk, Bunyami Ozogul, and Abdullah Kisaoglu. “Cecal Volvulus: A Rare Cause of Intestinal Obstruction.” The Eurasian Journal of Medicine 44.2 (2012): 115–116. PMC. Web. 8 May 2017.
- Lu, Teng, and Yi-mei Chng. “Adult Intussusception.” The Permanente Journal 19.1 (2015): 79–81. PMC. Web. 8 May 2017.
- Lindor, RA, MF Bellolio, AT Sadosty, F. Earnest, and D. Cabrera. “Adult Intussusception: Presentation, Management, and Outcomes of 148 Patients.” Medscape Log In. J Emerg Med, July 2012. Web. 13 May 2017.
- Waseem M, Rosenberg HK. Intussusception. Pediatr Emerg Care 2008;24:793–800.
- McClure, Scott. “Adult Intussusception: Not Like Trix, Not Just for Kids.” EmDOCs.net – Emergency Medicine Education. N.p., 10 June 2015. Web. 08 May 2017.
- Saverio, Salomone Di, Marco Bassi, Nazareno Smerieri, Michele Masetti, Francesco Ferrara, Carlo Fabbri, Luca Ansaloni, Stefania Ghersi, Matteo Serenari, Federico Coccolini, Noel Naidoo, Massimo Sartelli, Gregorio Tugnoli, Fausto Catena, Vincenzo Cennamo, and Elio Jovine. “Diagnosis and Treatment of Perforated or Bleeding Peptic Ulcers: 2013 WSES Position Paper.” World Journal of Emergency Surgery. BioMed Central, 03 Aug. 2014. Web. 13 May 2017.
- Hill, Andrew G. “Management of Perforated Duodenal Ulcer.” Surgical Treatment: Evidence-Based and Problem-Oriented. U.S. National Library of Medicine, 01 Jan. 1970. Web. 08 May 2017.
- Gaillard, Frank. “Perforated Duodenal Ulcer | Radiology Case.” Radiopaedia.org. N.p., n.d. Web. 13 May 2017.
- Butler KH, Fidler D, Grundmann K. Right lower quadrant abdominal pain in women of reproductive age: an algorithmic approach. Emergency Medicine Reports. 2002;1:1–13
- Curhan, Gary C., Mark D. Aronson, and Glenn M. Preminger. “Diagnosis and Acute Management of Suspected Nephrolithiasis in Adults.” UpToDate. N.p., Nov. 2015. Web. 13 May 2017.
- Colgan R, Williams M, Johnson JR. Diagnosis and treatment of acute pyelonephritis in women. Am Fam Physician. 2011;84:519–526
- Goonewardene, Sanchia, and Raj Persad. “Sterile Pyuria: A Forgotten Entity.” Therapeutic Advances in Urology 7.5 (2015): 295–298. PMC. Web. 8 May 2017.
- Long, Brit. “Pyelonephritis: It’s Not Always so Straightforward…” EmDOCs.net – Emergency Medicine Education. N.p., 21 Apr. 2017. Web. 08 May 2017.
- Barnes, David. “Gallbladder and Biliary Tract Disease.” Cleveland Clinic, Aug. 2010. Web. 08 May 2017.
- Walling, Anne. “Diagnosing Biliary Colic and Acute Cholecystitis.” Tips From Other Journals – American Family Physician. N.p., 15 Sept. 2000. Web. 13 May 2017.
- Bassiouny, Reem Hassan, and Amal Amin Abu El Maati. “Acute Right Lower Quadrant Pain beyond Acute Appendicitis: MDCT in Evaluation of Benign and Malignant Gastrointestinal Causes.” Acute Right Lower Quadrant Pain beyond Acute Appendicitis: MDCT in Evaluation of Benign and Malignant Gastrointestinal Causes. The Egyptian Society of Radiology and Nuclear Medicine, June 2014. Web. 08 May 2017.
- Lapsia, Snehal, and Sangeet Ghai. “Omental Infarction: A Rare Cause of Acute Abdominal Pain.” Emergency Medicine Journal : EMJ 24.11 (2007): 779. PMC. Web. 8 May 2017.
- Van Breda Vriesman, Adriaan, and Julien Puylaert. “Mimics of Appendicitis: Alternative Nonsurgical Diagnoses with Sonography and CT.” American Journal of Roentgenology: Vol. 186, No. 4 (AJR).” American Journal of Roentgenology, 2006. Web. 08 May 2017.









1 thought on “Appendicitis Mimics: ED Focused Management”
Pingback: Länkar v24 | Internmedicin