Authors: Tamar Reich, MD (EM Resident Physician, Einstein Medical Center Montgomery, East Norriton, PA) and Megan Stobart-Gallagher, DO (EM Residency Program Director and Attending Physician, Einstein Medical Center Montgomery, East Norriton, PA) // Reviewed by: Kayvan Moussavi, PharmD, BCCCP (Assistant Professor, Marshall B. Ketchum University College of Pharmacy); Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)
Case
A 55-year-old woman presents to the ED with chest pain and shortness of breath for the past 2 hours. She has a history of hypertension on lisinopril but no other outpatient medications or supplements. She underwent knee replacement surgery one week ago and was recently discharged from the hospital. She notes some swelling in her leg but thought that it was normal following her surgery. Her vital signs show HR 80, BP 140/90, RR 16, SpO2 97% on room air, oral temperature 37°C, weight 75 kg. She has normal renal function with a creatinine clearance of 55 ml/min. You send her for CTA. The CTA shows a subsegmental pulmonary embolism, with no signs of right heart strain.
Introduction
A previous emDocs post1 discussed the use of non-vitamin K oral anticoagulants (NOACs) and reversal of NOACs. NOACs, also known as direct-acting oral anticoagulants (DOACs), are commonly used for treatment of deep vein thrombosis (DVT),2 pulmonary embolism (PE),2 and stroke and systemic embolism prevention in patients with nonvalvular atrial fibrillation (i.e. atrial fibrillation in the absence of moderate to severe mitral stenosis or a mechanical heart valve).3 DOACs work by direct inhibition of thrombin (dabigatran) or factor Xa inhibition (rivaroxaban, apixaban, edoxaban).1,2 DOACs are generally noninferior or superior to warfarin (vitamin K antagonist) when comparing efficacy and safety (e.g. reduce risks of thromboembolism, cause less bleeding), are often easier to dose than warfarin, and typically do not require close laboratory monitoring.2,3 However, impaired renal function, impaired hepatic function,3drug-drug interactions,4,5 and certain medical conditions (e.g. anticoagulation requirements in patients with mechanical heart valve6) may preclude use.
There are many thrombotic diseases that require anticoagulation, including but not limited to, NSTEMI (non-ST elevation myocardial infarction),7 portal vein thrombosis,8 and non-bacterial thromboembolic endocarditis.9 However, there have not been adequate studies focused on use of DOACs in these populations. Therefore, this article will focus on DOACs for treatment of DVT and PE, and for stroke prevention in atrial fibrillation and atrial flutter.
There are several factors to consider when prescribing DOACs.10
- Age
- Body weight
- Renal function
- Liver function
- Comorbidities (e.g., cancer, pregnancy)
- Compliance and access to follow-up
- Risk factors for major bleeding
- Drug-drug interactions
Drugs that affect CYP3A4 or P-glycoprotein (e.g. clarithromycin, verapamil, phenytoin, rifampin) may affect serum levels of DOACs, so check for drug-drug interactions with resources such as Lexicomp or Micromedex before prescribing.4 Note that some drug-drug interactions may be severe enough to require consideration of alternative anticoagulants (e.g. warfarin may be required in patients taking rifampin due to severe interactions between DOACs and rifampin).
Patient conditions like cancer, impaired renal function, pregnancy, or breastfeeding may limit use of DOACs. For example, for treatment of venous thromboembolism (VTE), with:
- Gastrointestinal (GI) malignancy, use apixaban or low molecular weight heparin (LMWH)
- Severe renal impairment (creatinine clearance <30 mL/min), use warfarin (can consider apixaban but less evidence to support use)
- Pregnancy, use LMWH
- Breastfeeding, use warfarin or LMWH.11
Deep vein thrombosis
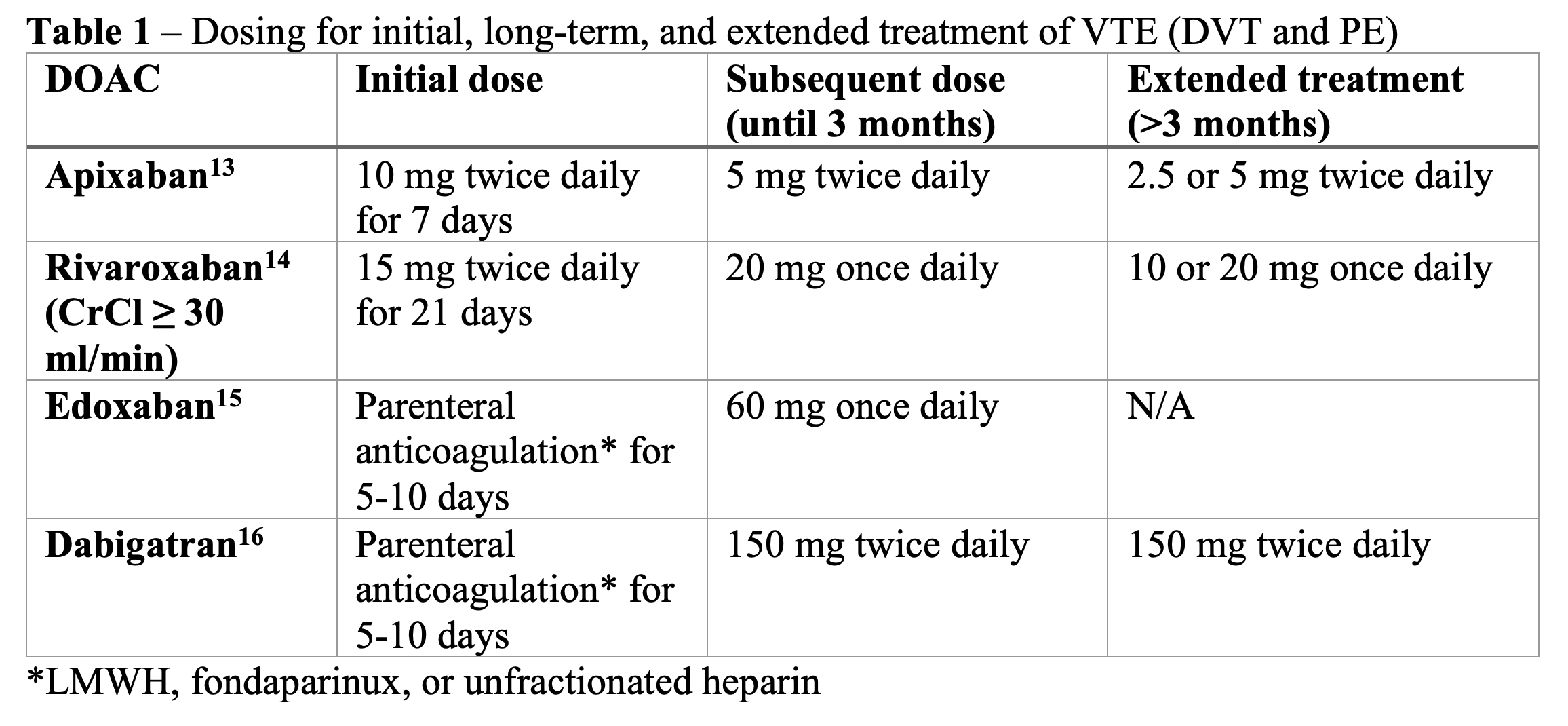
For patients who do not have a contraindication to DOAC use, DOACs are considered first-line treatment for DVT because they have lower rates of major bleeding than warfarin and have been shown to be non-inferior for treatment of DVT.11 Edoxaban and dabigatran require at least five days of parenteral anticoagulation prior to initiation,12 which may be more inconvenient for providers and patients. Rivaroxaban and apixaban do not require parenteral anticoagulation initially.12 See Table 1 for dosing regimens.
Treatment of acute DVT is typically for three months. There is an initial higher dose treatment period for 5-21 days followed by subsequent lower dose treatment period until three months, and extended treatment for more than three months.2
If it is an unprovoked DVT or provoked with a persistent risk factor, it is recommended to give extended treatment with DOAC. If provoked by a transient risk factor, extended treatment is not recommended.12 See Table 2 for examples of transient and persistent risk factors. There is no specified stop date for extended treatment, and it should be reassessed individually for each patient whether to continue treatment for prevention of DVT.2 A reduced dose of the DOAC should be considered for extended treatment.2,12
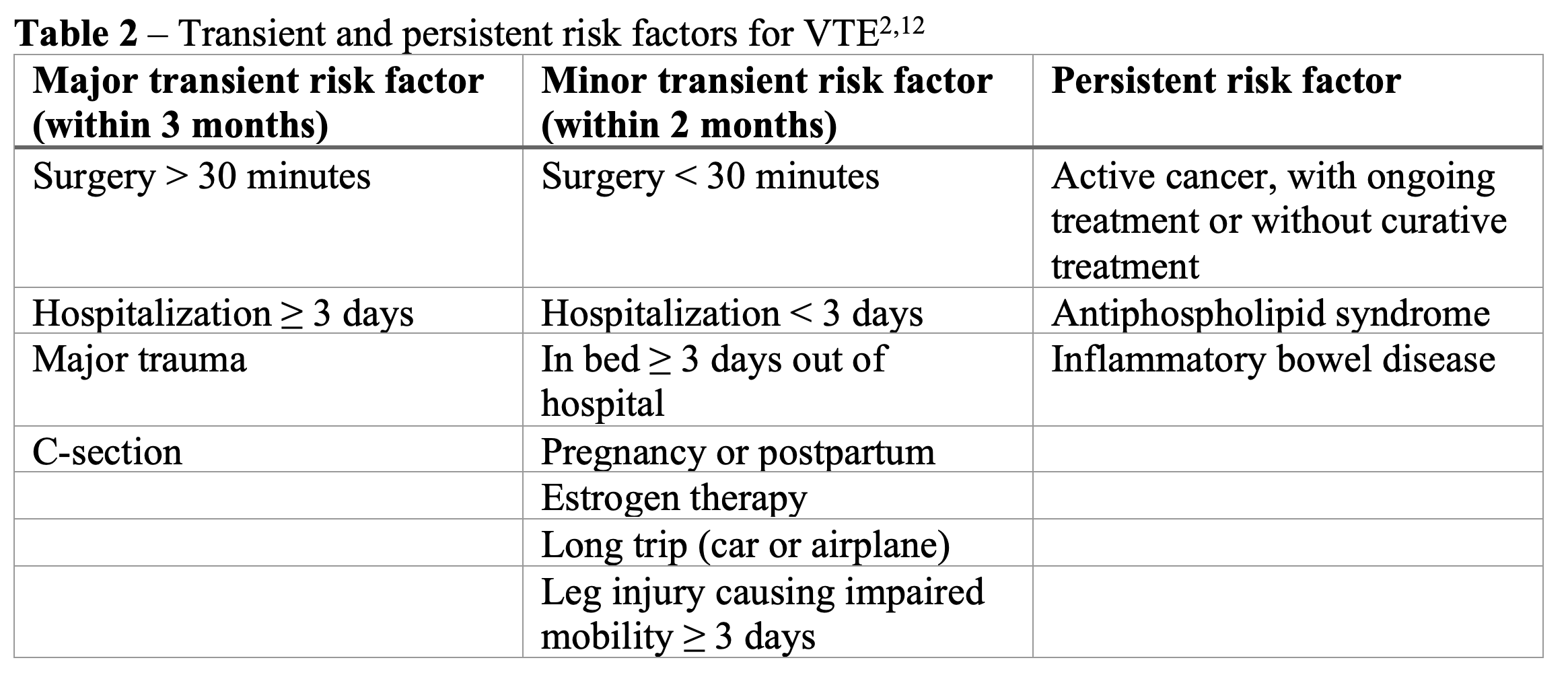
For an isolated distal DVT, the risk of progression or recurrence is low, so it does not always require anticoagulation.12 However, if the patient has severe symptoms or risk of extension, give anticoagulation.12 Some specific factors which might warrant anticoagulation include positive D-dimer, extensive thrombosis with clot >5cm long or >7mm wide, clot in multiple veins or near proximal veins, patient has active cancer, history of VTE, or COVID-19 (see CHEST guidelines for complete list of criteria).2 If the patient is not receiving anticoagulation, they should undergo serial imaging for two weeks. Serial imaging recommendations are to perform a repeat ultrasound between 1 to 3 days, and then again between 7 to 10 days after initial diagnosis.12,17
The 2021 updated CHEST guidelines for treatment of VTE2 recommend treatment of superficial venous thrombosis (SVT) in certain cases, but first-line treatment is with fondaparinux 2.5 mg subcutaneously daily for 45 days. If the patient is unable to use parenteral treatment, rivaroxaban 10 mg daily for 45 days is recommended. Superficial venous thrombosis should be treated if it is extensive, above the knee, involving the greater saphenous vein, or if the patient has cancer, recent surgery, history of VTE or SVT, or severe symptoms.2
Pulmonary embolism
A retrospective cohort study18 found that the rate of discharge for PE is less than five percent, but we could be discharging a larger percentage now with use of DOACs. Although almost 20% of patients discharged with PE return to the ED within 30 days, only about 10% end up being hospitalized.18 Up to 50% of patients may be low risk and could be discharged with outpatient treatment.19
We can use decision tools to find which patients are low risk and can be discharged. Some tools include PESI (pulmonary embolism severity index), sPESI (simplified pulmonary embolism severity index), and Hestia Criteria, which are used to predict mortality rates and have been validated for use.20,21,22 Therefore, if the patient is hemodynamically stable and has negative biomarkers, these decision tools can be used to risk-stratify patients diagnosed with PE.19 For example, sPESI includes criteria as follows: age (>80), heart rate (≥110), systolic blood pressure (<100), oxygen saturation (<90%), history of cancer, heart failure, or chronic pulmonary disease.21 If the sPESI score is ≥ 1, the patient is considered high risk, and consideration should be given for admission for parenteral treatment.19,21
If the decision tool determines that the patient is low-risk for outpatient treatment, and the patient will be compliant and is able to take the medication, use the HAS-BLED or Outpatient Bleeding Risk Index to determine risk of bleeding.19 If the HAS-BLED score is 3 or more, the patient is considered high-risk for bleeding; starting outpatient anticoagulation should be reconsidered, and any reversible risk factors corrected.23 If the Outpatient Bleeding Risk Index is 0, the patient is considered low-risk for bleeding events and outpatient management can be initiated with a DOAC.24
Recommended treatment duration for PE is three months.2 Similar to DVT treatment, rivaroxaban and apixaban can be initiated in low-risk PE and do not require parenteral treatment initially (unlike edoxaban and dabigatran) (Table 1). DOACs should be avoided in pregnancy12 and in most patients with antiphospholipid syndrome.25 In patients with gastrointestinal malignancy, apixaban or LMWH can be used. In patients with non-GI cancer, DOACs are still recommended over LMWH.2,11 Patients with morbid obesity (BMI >40 or weight >120kg) will require close monitoring but DOACs can still be utilized.26
Updated CHEST guidelines2 recommend treatment for certain patients with subsegmental PE without DVT. The criteria include patients who have active cancer, pregnancy, are hospitalized or have reduced mobility, or who have no reversible risk factor that may have triggered the PE.2,27 In addition, a recent study28 found that patients without the above risk factors, but who had multiple subsegmental PEs or who were older than 65 years old had higher rates of recurrent VTE. Therefore, although this was not included in the CHEST guidelines, it should be considered in patients with subsegmental PE when considering whether to start anticoagulation in the ED.29
Atrial fibrillation and atrial flutter
In both atrial fibrillation and atrial flutter, anticoagulation is important for preventing stroke and thromboembolism.3,30 It is also important to use anticoagulation around the time of cardioversion for those patients who are undergoing cardioversion.3 In patients that require oral anticoagulation for nonvalvular atrial fibrillation and atrial flutter, DOACs are recommended over warfarin, except for certain circumstances (e.g. recent implantation of bioprosthetic valve within 3-6 months, drug-drug interaction, patients unable to comply with dosing or afford the medication).4 As mentioned above, nonvalvular atrial fibrillation is defined as atrial fibrillation without moderate or severe mitral stenosis and without mechanical heart valves.3 Patients with valvular atrial fibrillation should receive anticoagulation with warfarin.3
For patients with bioprosthetic valves, studies have shown non-inferiority of DOACs, specifically rivaroxaban, edoxaban, dabigatran, and apixaban, compared to warfarin.31-34 Furthermore, a recent meta-analysis showed noninferior or superior effectiveness and safety of DOACs compared to warfarin for preventing stroke or embolism in patients with atrial fibrillation and bioprosthetic heart valves.35 A portion of the studies only included patients who had undergone valve repair at least 3 months earlier, so some guidelines only recommend DOACs more than 3 months following bioprosthetic valve implantation.35
DOAC prescription in the ED for atrial fibrillation and atrial flutter is most relevant when patients are discharged after cardioversion or discharged with rate control and plan for future cardioversion. The Ottawa Aggressive Protocol36describes a process for safe cardioversion of patients in the ED. A more recent paper describes “The Iowa less aggressive protocol,” which integrates cardiac CT to ensure that patients do not have a left atrial thrombus prior to cardioversion, specifically for patients who have atrial fibrillation > 48 hours or high risk of thromboembolism and atrial fibrillation for > 12 hours.37
Prior to prescribing a DOAC on discharge from the ED, calculate the CHA2DS2-VASc score to determine the patient’s risk of stroke.30 The components are as follows (each 1 point except if marked otherwise):
- Congestive heart failure
- Hypertension
- Age ≥75 years (2 points)
- Diabetes mellitus
- Stroke/TIA/thromboembolism (2 points)
- Vascular disease
- Age 65 to 74 years
- Sex category (female sex)30
If the score is ≥2 for males or ≥3 for females, the patient should receive long-term anticoagulation.30 If the score is 1 for males and 2 for females, and one of these points is due to the patient being 65-74 years old, long-term anticoagulation is also recommended.30 This is because age is considered a higher risk factor for stroke than the other risk factors that are assigned one point; age is the biggest risk factor after history of previous stroke.38 Otherwise, for patients with a score of 1 for males and 2 for females who are < 65 years old, the decision to anticoagulate may depend on the patient’s frequency of atrial fibrillation,30 and anticoagulation should be considered for these patients.3 For a score of 0 in males or 1 in females, anticoagulation is not recommended, but there can be joint decision-making with the patient based on their preferences and bleeding risk.30
After calculating the CHA2DS2-VASc score, calculate the HAS-BLED score, which helps prognosticate the risk of bleeding and includes the following components, which are each assigned one point unless indicated:
- Hypertension (systolic blood pressure > 160 mmHg)
- Abnormal renal function (chronic dialysis, kidney transplant, or creatinine ≥ 200 µm/L or 2.26 mg/dL) and/or liver function (cirrhosis, or bilirubin > 2 times upper limit of normal and AST, ALT, or alkaline phosphatase > 3 times upper limit of normal) – 1 or 2 points
- Stroke history
- Bleeding history or predisposition (bleeding requiring hospitalization or transfusion, or chronic bleeding disorder)
- Labile INRs, for patients taking warfarin (unstable INRs, very high INRs, or in therapeutic range less than 60% of the time)
- Elderly (age > 65 years)
- Drugs (aspirin or NSAIDs) and/or excess alcohol use – 1 or 2 points30
The total score then can be used to find the risk of bleeding as a rate in one hundred patient-years by referencing the source table.30
For patients with atrial fibrillation or atrial flutter which has been occurring for ≥48 hours or with unknown duration, the patient should receive anticoagulation with either warfarin or a DOAC for 3 weeks before and at least 4 weeks after cardioversion.3 Guidelines say that transesophageal echo (TEE) can be done to check for an atrial thrombus;3,30 if there is no thrombus, cardioversion can be done immediately if they are first anticoagulated and then the anticoagulation is continued for 4 weeks.3 Similarly, if the patient needs cardioversion immediately because they are hemodynamically unstable, start anticoagulation immediately and continue it for 4 weeks.3
In atrial fibrillation or atrial flutter <48 hours, if CHA2DS2-VASc score is ≥2 for males or ≥3 for females, give anticoagulation (heparin or DOAC) as soon as possible, in addition to long-term coagulation after cardioversion.3 If CHA2DS2-VASc score is 0 for males or 1 for females, you can consider anticoagulation before cardioversion, but do not need to continue this long-term afterwards.3
One other point to note is that when patients with atrial fibrillation who are not already on anticoagulation present with transient ischemic attack, if CT is negative, you can consult neurology regarding starting anticoagulation with a DOAC (or warfarin). If there was an ischemic stroke found on CT, you should follow hospital guidelines for administration of thrombolysis but heparin or a DOAC should be started later during their hospital stay.39
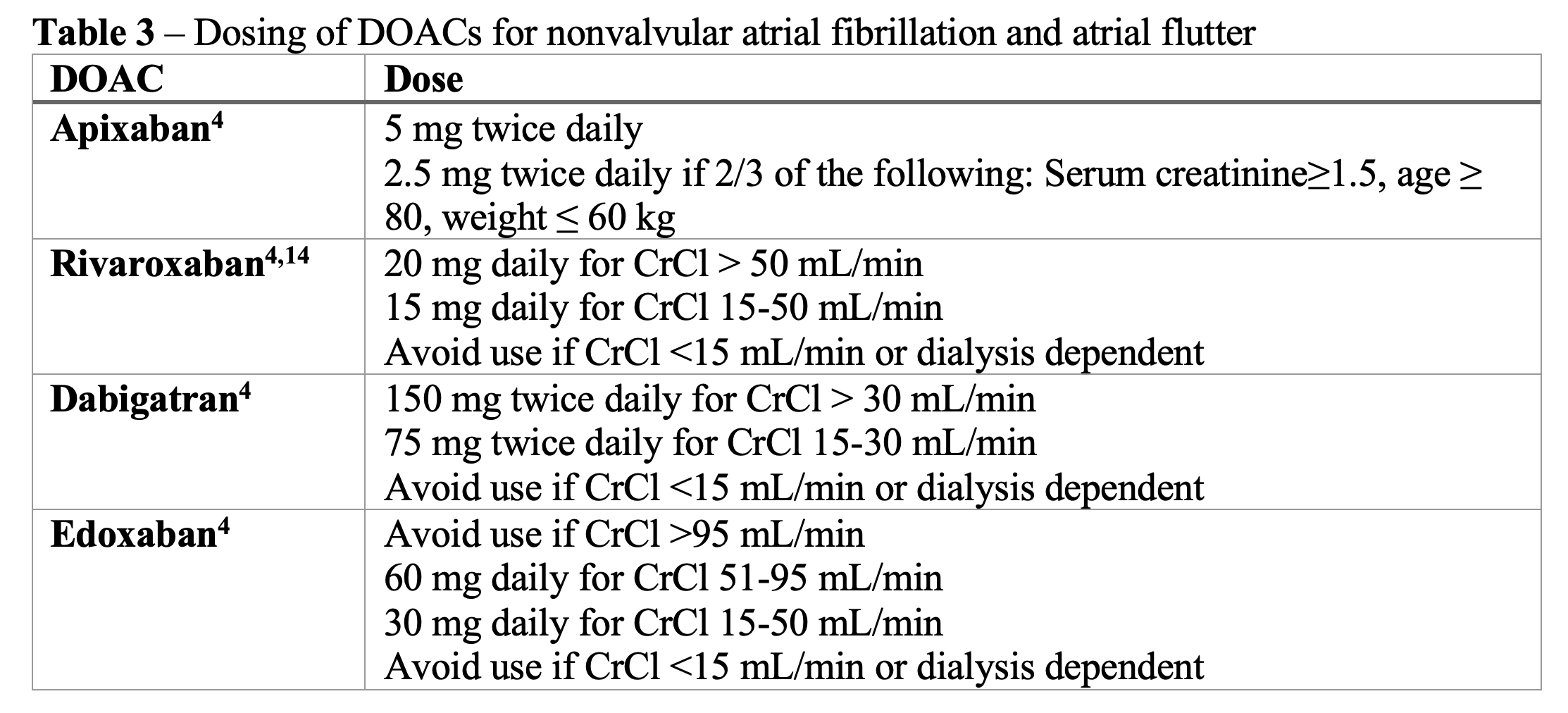
The dosing for DOACs in atrial fibrillation/atrial flutter is different from the dosing for the treatment for VTE. The typical dosing regimens can be found in Table 3 below. The doses may need to be adjusted for renal impairment or for other factors (e.g., age, weight), so be sure to look up the correct dose if the patient has CKD, is ≥ 80 years old, or weighs ≤ 60 kg. As mentioned above, also ensure that patients with morbid obesity have close follow-up to assess if the DOAC dose is adequate or if they need to switch to warfarin.26
Take-home points
- DOACs are recommended instead of warfarin for VTE treatment and stroke prevention in atrial fibrillation/atrial flutter.
- Although DOACs are safer and have similar efficacy to warfarin, there are many contraindications that may preclude use (e.g., severe kidney or liver dysfunction, pregnancy, breastfeeding, GI malignancy, drug-drug interactions). Ensure your patient does not have any contraindications to DOACs before prescribing.
- Appropriate DOAC dosing may require adjustments based on age, body weight, renal function, or other factors. Check for correct dose before prescribing.
- Always balance the risk of bleeding with the benefits of oral anticoagulation. You can use the HAS-BLED or Outpatient Bleeding Risk Index to assess the risk of bleeding and use the CHA2DS2-VASc score in atrial fibrillation to assess the need for anticoagulation.
- Use a clinical decision tool like PESI, sPESI, and Hestia Criteria to determine if a patient is low risk for being discharged with outpatient treatment for PE.
Case Conclusion
You use the PESI score to determine that your patient is low risk for discharge with outpatient PE treatment. Her PESI score is 55 (due to her age, with no points given for male sex, history of cancer, heart failure, chronic lung disease, abnormal vital signs, or altered mental status), which places her in the Class I or “Very Low” risk category.20 In addition, her Outpatient Bleeding Risk Index is 0. This is because she has none of the following risk factors: age ≥ 65, history of stroke or GI bleed, or other conditions including severe anemia, kidney impairment, diabetes, or recent myocardial infarction.24
Because she has reduced mobility from her recent surgery, you decide to treat her subsegmental PE. You also perform a lower extremity ultrasound and find that she has concurrent DVT, further supporting your decision to anticoagulate. Because the DVT was triggered by a major transient risk factor, which is her surgery lasting more than 30 minutes in addition to a hospitalization for 3 days, she will not require extended anticoagulation for more than 3 months. You prescribe rivaroxaban 15 mg bid for 21 days, then 20 mg daily for 3 months. This choice of medication was used to avoid the need for initial parenteral treatment (which would be required for edoxaban or dabigatran), her creatinine clearance is > 30 ml/min, she does not have a GI malignancy, and there is no drug interaction with lisinopril or other factors limiting her use of DOACs. She follows up with her PCP and successfully completes the treatment with no major bleeding events.
References
- Pickens A. EM in 5: NOACs – Novel Oral Anticoagulants. net – Emergency Medicine Education.http://www.emdocs.net/emin5-noacs-novel-oral-anticoagulants/. Published March 19, 2018. Accessed July 24, 2022.
- Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: Second update of the CHEST guideline and expert panel report. Chest. 2021;160(6):E545-E608. doi:10.1016/j.chest.2021.07.055
- January C, Wann L, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2019 Jul, 74 (1) 104–132. https://doi.org/10.1016/j.jacc.2019.01.011
- Manning WJ, Singer DE, Lip GYH. Atrial fibrillation in adults: Use of oral anticoagulants. https://www.uptodate.com/contents/atrial-fibrillation-in-adults-use-of-oral-anticoagulants. Published May 16, 2022. Accessed August 14, 2022.
- Mar PL, Gopinathannair R, Gengler BE, et al. Drug interactions affecting oral anticoagulant use. Circulation: Arrhythmia and Electrophysiology. 2022;15. https://doi.org/10.1161/CIRCEP.121.007956
- Biase LD. Use of direct oral anticoagulants in patients with atrial fibrillation and valvular heart lesions. Journal of the American Heart Association. 2016;5:e002776. https://doi.org/10.1161/JAHA.115.002776
- Kapoor S, Zeserson E. Current ED management of non-ST segment elevation MI (NSTEMI): A practice update. net – Emergency Medicine Education. http://www.emdocs.net/9047-2/. Published July 30, 2016. Accessed July 24, 2022.
- Hanlin ER, Kaelin A. Portal vein thrombosis: A primer for emergency medicine physicians. net – Emergency Medicine Education. http://www.emdocs.net/portal-vein-thrombosis-primer-emergency-medicine-physicians/. Published December 27, 2017. Accessed July 24, 2022.
- Dirkes W, Kessler J, Baker J, Wedmore I. A case of non-bacterial thromboembolic endocarditis. net – Emergency Medicine Education. http://www.emdocs.net/nonbacterial-thromboembolic-endocarditis/. Published June 23, 2016. Accessed July 24, 2022.
- Helman A, Himmel W, Douketis J, Bell B. DOACs part 1: Use and misuse. Emergency Medicine Cases.https://emergencymedicinecases.com/doacs-use-misuse/. Published November 2016. Accessed July 13, 2022.
- Chopard R, Albertsen IE, Piazza G. Diagnosis and treatment of lower extremity venous thromboembolism: A review. 2020;324(17):1765–1776. doi:10.1001/jama.2020.17272
- Management of deep vein thrombosis (DVT). EBSCO Information Services.https://www.dynamed.com/management/management-of-deep-vein-thrombosis-dvt. Accessed August 9, 2022.
- IBM Micromedex® DRUGDEX®. Apixaban. IBM Watson Health/EBSCO Information Services. https://www.dynamed.com/drug-monograph/apixaban-12. Accessed August 14, 2022.
- IBM Micromedex® DRUGDEX®. Rivaroxaban. IBM Watson Health/EBSCO Information Services. https://www.dynamed.com/drug-monograph/rivaroxaban. Accessed August 14, 2022.
- IBM Micromedex® DRUGDEX®. Edoxaban. IBM Watson Health/EBSCO Information Services. https://www.dynamed.com/drug-monograph/apixaban-12. Accessed September 7, 2022.
- IBM Micromedex® DRUGDEX®. Dabigatran. IBM Watson Health/EBSCO Information Services. https://www.dynamed.com/drug-monograph/apixaban-12. Accessed September 7, 2022.
- Needleman L, Cronan JJ, Lilly MP, et al. Ultrasound for lower extremity deep venous thrombosis: Multidisciplinary recommendations from the society of radiologists in ultrasound consensus conference. Circulation. 2018;137:1505–1515. https://doi.org/10.1161/CIRCULATIONAHA.117.030687
- Westafer LM, Shieh MS, Pekow PS, Stefan MS, Lindenauer PK. Outpatient management of patients following diagnosis of acute pulmonary embolism. Acad Emerg Med. 2021;28(3):336-345. doi:10.1111/acem.14181
- Long B, Koyfman A. Outpatient PE management: Controversies, pearls, and pitfalls. net – Emergency Medicine Education. http://www.emdocs.net/outpatient-pe-management-controversies-pearls-pitfalls/. Published November 18, 2016. Accessed August 9, 2022.
- Zhou XY, Ben SQ, Chen HL, Ni SS. The prognostic value of pulmonary embolism severity index in acute pulmonary embolism: a meta-analysis. Respir Res. 2012;13(1):111. Published 2012 Dec 4. doi:10.1186/1465-9921-13-111
- Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med.2010;170(15):1383–1389. doi:10.1001/archinternmed.2010.199
- Zondag W, Vingerhoets LM, Durian MF, et al. Hestia criteria can safely select patients with pulmonary embolism for outpatient treatment irrespective of right ventricular function. J Thromb Haemost. 2013;11(4):686-692. doi:10.1111/jth.12146
- Kooiman J, van Hagen N, Iglesias Del Sol A, et al. The HAS-BLED score identifies patients with acute venous thromboembolism at high risk of major bleeding complications during the first six months of anticoagulant treatment. PLoS One. 2015;10(4):e0122520. Published 2015 Apr 23. doi:10.1371/journal.pone.0122520
- Wells PS, Forgie MA, Simms M, et al. The outpatient bleeding risk index: Validation of a tool for predicting bleeding rates in patients treated for deep venous thrombosis and pulmonary embolism. Arch Intern Med.2003;163(8):917–920. doi:10.1001/archinte.163.8.917
- Pastori D, Menichelli D, Cammisotto V, et al. Use of direct oral anticoagulants in patients with antiphospholipid syndrome: A systematic review and comparison of the international guidelines. Front Cardiovasc Med. 2021;8:715878. doi:10.3389/fcvm.2021.715878
- Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016 Jun;14(6):1308-13. doi: 10.1111/jth.13323
- Smith C. Update on antithrombotic therapy for VTE disease. Journal Feed. https://journalfeed.org/article-a-day/2022/update-on-antithrombotic-therapy-for-vte-disease/. Published January 26, 2022. Accessed July 24, 2022.
- Le Gal G, Kovacs MJ, Bertoletti L, et al. Risk for recurrent venous thromboembolism in patients with subsegmental pulmonary embolism managed without anticoagulation: A multicenter prospective cohort study. Ann Intern Med. 2022;175(1):29-35. doi:10.7326/M21-2981
- Parnell S, Stubblefield B. Subsegmental PE – small but significant? Journal Feed. https://journalfeed.org/article-a-day/2022/subsegmental-pe-small-but-significant/. Published January 11, 2022. Accessed August 14, 2022.
- Manning WJ, Singer DE, Lip GYH. Atrial fibrillation in adults: Selection of candidates for anticoagulation. https://www.uptodate.com/contents/atrial-fibrillation-in-adults-selection-of-candidates-for-anticoagulation. Published May 19, 2022. Accessed August 14, 2022.
- Guimarães HP, Lopes RD, de Barros E Silva PGM, et al. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N Engl J Med. 2020;383(22):2117-2126. doi:10.1056/NEJMoa2029603
- Carnicelli AP, De Caterina R, Halperin JL, et al. Edoxaban for the prevention of thromboembolism in patients with atrial fibrillation and bioprosthetic valves. Circulation. 2017;135(13):1273-1275. doi:10.1161/CIRCULATIONAHA.116.026714
- Durães, A.R., de Souza Roriz, P., de Almeida Nunes, B. et al. Dabigatran versus warfarin after bioprosthesis valve replacement for the management of atrial fibrillation Postoperatively: DAWA Pilot Study. Drugs R D. 2016;16:149–154. https://doi.org/10.1007/s40268-016-0124-1
- Guimarães PO, Pokorney SD, Lopes RD, et al. Efficacy and safety of apixaban vs warfarin in patients with atrial fibrillation and prior bioprosthetic valve replacement or valve repair: Insights from the ARISTOTLE trial. Clin Cardiol. 2019;42(5):568-571. doi:10.1002/clc.23178
- Cao Y, Zheng Y, Li S, et al. An Updated Meta-Analysis of DOACs vs. VKAs in atrial fibrillation patients with bioprosthetic heart valve. Front Cardiovasc Med. 2022;9:899906. doi:10.3389/fcvm.2022.899906
- Stiell IG, Clement CM, Perry JJ, et al. Association of the Ottawa Aggressive Protocol with rapid discharge of emergency department patients with recent-onset atrial fibrillation or flutter. Canadian Journal of Emergency Medicine. 2010;12(3):181-191. doi:10.1017/S1481803500012227
- Christians BE, Solie CJ, Swanson MB, et al. The Iowa less aggressive protocol: A mixed-methods study on the novel treatment protocol of atrial fibrillation. Am J Emerg Med. 2021;45:439-445. doi:10.1016/j.ajem.2020.09.046
- Lee CJ, Toft-Petersen AP, Ozenne B, et al. Assessing absolute stroke risk in patients with atrial fibrillation using a risk factor-based approach. Eur Heart J Cardiovasc Pharmacother. 2021;7(FI1):f3-f10. doi:10.1093/ehjcvp/pvaa063
- Helman A. EM cases: TIA update – Risk stratification, workup and DAPT. net – Emergency Medicine Education. http://www.emdocs.net/em-cases-tia-update-risk-stratification-workup-and-dapt/. Published January 18, 2019. Accessed July 24, 2022.








