Author: Vincent R. Lee, MD (@vinleetox, Senior Medical Toxicology Fellow, Division of Medical Toxicology, Department of Emergency Medicine Northwell Health, Manhasett, NY) and Joshua N. Nogar MD (Medical Toxicology Fellowship Program Director, Division of Medical Toxicology, Department of Emergency Medicine Northwell Health, Manhasett, NY) // Reviewed by: Cynthia Santos, MD (@CynthiaSantosMD); Alex Koyfman, MD (@EMHighAK); Manny Singh, MD (@MPrizzleER); and Brit Long, MD (@long_brit)

Introduction:
Currently, there are no U.S. Food and Drug Administration (FDA) approved medications or best available treatment for COVID-19. The management mainly involves meticulous infection-control for prevention and aggressive supportive care. Given the rapid escalating pandemic, healthcare providers have been administering experimental medications based on in-vitro and anecdotal clinical data in the hopes they will prove effective for hospitalized patients [1].
The antimalarial drugs, chloroquine and hydroxychloroquine, have been widely advertised as a potential treatment, despite the lack of peer-reviewed high-quality studies [2]. Just a few days after media coverage, one fatality and one hospitalization were reported in the U.S. after ingestion of anti-parasitic fish powder containing chloroquine phosphate for prophylaxis [3] and three hospitalizations of poisonings after self-treatment were reported in Lagos, Nigeria [4]. On March 28th, the FDA issued an emergency-use-authorization, allowing for the distribution of antimalarial drugs (chloroquine and hydroxychloroquine) from the Strategic National Stockpile to hospitals [5]. The surge in stockpiling and prescription demand by hospitals and providers have already created a national shortage in the U.S. [6]. With the world’s renewed interest in chloroquine, clinicians need to be cognizant of the potential for significant toxicity in the midst of the COVID-19 pandemic.
Background
Chloroquine’s claim to fame as an anti-malarial earned its designation the World Health Organization’s (WHO) list of essential medicines, despite its narrow-theraputic index. For decades, its was a front-line drug for the treatment and prophylaxis of malaria, but its use gradually declined due to chloroquine-resistant Plasmodium falciparum. Hydroxychloroquine, chloroquine’s synthetic analog, is more commonly used today because of its reduced toxicity (about 40%) without an proportionate lost of therapeutic activity [6]. Other off-label uses of hydroxychloroquine include treatment of autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus.
The clinical studies on chloroquine toxicity and it’s management were largely derived from intentional ingestions from France. However since the 1970s, chloroquine has been one of the most common causes of overdoses in Africa, Asia, and the South Pacific regions, since it is easily accessible without a prescription at a relatively low cost [7, 8]. In the 1980s, France experienced a cluster of poisonings owing to the book “Suicide Mode d’Emploi” promoting chloroquine overdose as a means of suicide [9 – 11].
Rationale for COVID-19 (Figure 1) [12, 13]
- Blocks virus infection by increasing endosomal & lysosomal pH, altering the conditions required for virus-to-host cell fusion.
- Interferes with glycosylation of ACE2-receptors on the virus, preventing virus-to-host cell fusion.
- Autophagy inhibitor which modulates acidification of endosomes -> inhibiting formation of autophagosomes -> preventing virus-to-host cell fusion.
- Inhibits virus replication via reduction of mitogen-activated protein (MAP) kinase activation.
- Alters M-protein maturation and interfere with viron assembly and budding.
- Immune-modulating activity contributing to anti-inflammatory response, possibly reduction of cytokine storm.
- Inhibits locomotion of neutrophils and chemotaxis of eosinophils.
- Impairs complment-dependent antigen-antibody reactions.
Figure 1. Schematic representation of the possible effects of chloroquine on SARS-CoV-2 replcation cycle. Obtained directly from Devaux et al. 2020.

Where is the evidence for COVID-19 treatment?
Please see this emDocs post for further information on these medications for COVID-19. The current literature has many limitations including lack of comparator groups, small sample sizes, and significant biases.
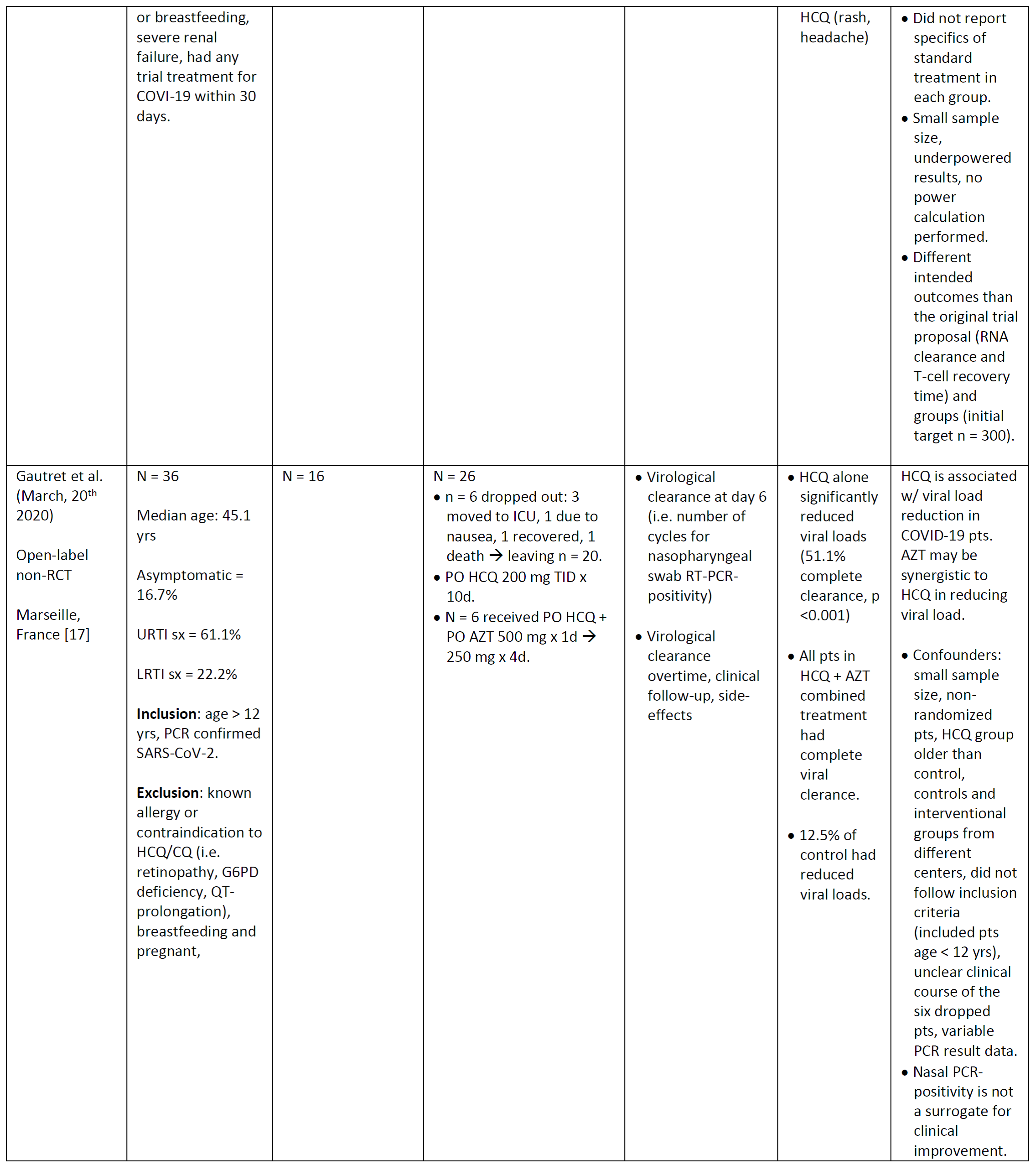
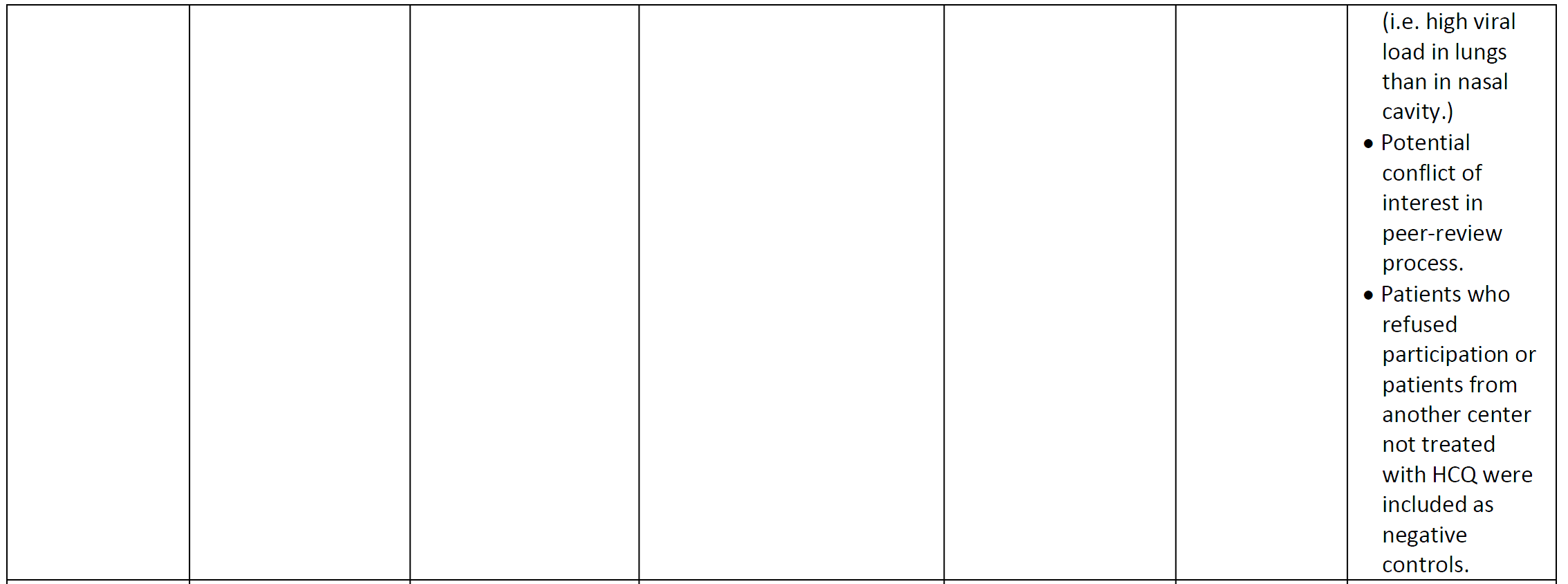
Table 1. Characterisitics of literature studying the efficacy and safety of chloroquine or hydroxychloroquine in patients with COVID-19. CQ = chloroquine, HCQ = hydroxychloroquine, AZT = azithromycin, d = days, PNA = pneumonia, yrs = years, sx = symptoms

Pharmacokinetics/Toxicokinetics [14]
Chloroquine
- High volume of distribution (Vd) & mildly protein bound.
- Exhibits slow distribution from blood compartment to central compartment, resulting in transiently high whole blood concentrations shortly after ingestion.
- Absorption is rapid and almost complete with high oral bioavailability.
- Metabolism: Hepatic to main metabolite desethylchloroquine.
- Time to peak serum concentration (Tmax) = Oral: 1.5 – 2 hours
- Half-life (t1/2) = 3-5 days
- Excretion: renally, theoretically enhanced with urinary acidification.
Hydroxychloroquine
- High Vd & mildly protein bound (mostly to albumin).
- Absorption: Incomplete and variable ranging from 25-100%.
- Metabolism: Hepatic to active metabolites bidesethylchloroquine, desethylhydroxychloroquine, & desethylchloroquine.
- T1/2= 40 days
- Excretion: renally, theoretically enhanced with urinary acidification.
Pathophysiology [14-23]
- Acts as a Vaughan Williams Class Ia antidysrhythmic with “Quinidine-like” effect.
- Sodium channel blockade
- May lead to widened QRS-interval and at risk for developing tachydysrhythmias.
- Potassium channel blockade
- May lead to prolonged QT-interval and at risk for developing Torsade de pointes (TdP).
- Increased insulin release leading to hypoglycemia and hypokalemia.
- Hypokalemia results from potassium shift from the extracellular to intracellular compartments.
- Overall: Intermediate prolongation of the action potential, negative inotropy (Figure 2).
- Sodium channel blockade

Figure 2. Effect of Class 1a antidysrhythmics on the action potential. Red arrow showing right-ward shift at phase 0 of depolarization.
- Drug binds to melanin in the retinal pigment epithelium (RPE), causing damage to the macular cones outside the fovea. Characteristically, begins with photoreceptor thinning appearing as a parafoveal ring with progression to a visible bull’s eye retinopathy (Figure 3C) when the RPE becomes damaged [21].

Figure 3. Comparison of normal fundus and hydroxychloroquine retinopathy patterns via ultra-widefield autofluorescence. Obtained directly from Melles et al. (2015) [21]. Legend for images. A: Normal, B: Parafoveal pattern (i.e. Bull’s eye pattern), C: Mixed pattern, and D: pericentral pattern.
Proposed Experimental Dosages for COVID-19
- PO Hydroxychloroquine sulfate
- 200 mg TID x 10 days [16].
- 100-200 mg BID x 5-14 days [17].
- 400 mg daily x 5 days [17, 18].
- 400 mg BID on day 1, then 200 mg BID on days 2-5 [19].
- PO Chloroquine phosphate
- 500 mg BID x 10 days [17].
- 500 mg BID x 7 days (adults > 50 kg); 500 mg BID on days 1-2, then 500 mg daily on days 3-7 (for adults < 50 kg) [20].
- Initially 1000 mg then 500 mg 12 hours later on day 1, then 500 mg BID on days 2-5 [17].
Therapeutic dosing: Adverse effects [14, 24]
- Gastrointestinal: May cause hypersensitivity hepatitis with increased liver-function enzymes, nausea/vomiting, abdominal cramps/pain.
- Hematologic: Hemolytic anemia in glucose-6-phophate dehydrogenase (G6PD) deficiency patients, agranulocytosis, and thrombocytopenia.
- Ocular/Ear disorders: Chronic and/or high-dose therapy has been associated with retinopathy, sensorineural deafness and tinnitus.
- Musculoskeletal: Rare muscular weakness or myopathy.
- Metabolic: Hypoglycemia.
- Immunologic: Hypersensitivity reactions such as myocarditis.
- CNS: Increased risk of seizures in those with epilepsy.
Diagnostics [19, 20, 23]
- Before starting therapeutic treatment, consider possible benefits, risks, and contraindications:
- Asian Race may increase risk for peripheral retinopathy.
- Kidney and/or liver disease may predispose to toxicity.
- History of G6PD-deficiency.
- Prescense of retinal or visual field changes of any etiology.
- History of cardiac disease, uncorrected hypokalemia and/or hypomagenesemia, or bradycardia (HR < 50 bpm).
- Drug-drug interactions
- Concomitant QT-prolonging medications.
- HCQ and CQ are potent CYP2D6 inhibitors (i.e. may raise metoprolol levels).
Clinical Manifestations of Toxicity
Severe chloroquine poisoning is associated with: [14]
- Ingestions of > 5 grams for adults and > 1 gram for children
- Systolic Blood Pressure (SBP) < 80 mm Hg
- QRS-Interval > 120 msec
- Ventricular Fibrillation
Signs and Symptoms [14]
- Time to onset of symptoms is about 1-3 hours post-ingestion.
- Respiratory depression is common.
- May progress rapidly to apnea, hypotension, and cardiovascular (CV) collapse.
- EKG abnormalities: widened QRS-interval, AV block, ST-T wave depressions, U waves, QT-interval prolongation, and TdP.
- Neurological: CNS depression, dizziness, headache, seizures, and transient parkinsonism.
- Ophthalmic: Peripheral retinopathy and loss of color vision is more associated with chronic use.
Management [14]
a) Gastrointestinal Decontamination:
- Be very cautious with giving 1 g/kg of PO activated charcoal because severe toxicity is associated with rapid CNS depression, seizures, and CV collapse. These patients are high-risk for fatal-aspiration charcoal pneumonitis.
- Try to estimate the dose ingested!
- If life-threatening ingestion of > 5 grams in adult or > 1 gram in child, consider protecting the airway with early intubation and orogastric lavage.
b) Early aggressive supportive care [14, 25, 26, 31]
- Serial blood glucose measurements for monitoring hypoglycemia
- For severe toxicity (i.e. apneic, hypotension, cardiovascular collapse, dysrhythmias), consider:
- EARLY endotracheal intubation and mechanical ventilation (Note: avoid barbituates for induction as may cause sudden cardiac arrest). [10]
- High-dose IV epinephrine at 0.25 mcg/kg/min with increasing by 0.25 mcg/kg/min until SBP > 90 mm Hg or MAP > 65 mm Hg. (normal dose range: 1-10 mcg/min).
- Note: Epinephrine may exacerbate pre-existing hyperkalemia.
- High-dose IV diazepam at 2 mg/kg over 30 minutes then 1-2mg/kg/day for 2-4 days.
- Note: May exhaust your hospital’s supply of diazepam.
Note: Combining early mechanical ventilation with administration of high-dose diazepam and high-dose epinephrine showed potential benefit with less cardiovascular toxicity [10].
- Diazepam is believed to have a central antagonistic effect, anticonvulsant effect, antidysrhythmic effect and interaction inverse to chloroquine/hydroxychloroquine, and decrease in chloroquine & hydroxychloroquine induced-vasodilation.
- Watch out for the knee-jerk reflex for treating a widened-QRS!
- Sodium bicarbonate treatment controversial since it may exacerbate concomitant severe-hypokalemia.
- Consider giving 1-2 mEq/kg IVP NaHCO3 in combination with evaluating the patient’s degree of cardiotoxicity and hypokalemia.
- Should I replete potassium? Not as straightforward…
- Potassium supplementation is reasonable for severe hypokalemia (< 1.9 mEq/L) to prevent worsening QT-prolongation and precipitation of TdP.
- If supplementing, it is critical to anticipate rebound hyperkalemia as toxicity resolves with the redistribution of potassium from the intracellular space to extracellular.
- Any role for Intralipids?
- Theoretically, intralipids should act as an “lipid-sink” since chloroquine is lipophilic. However, there is no evidence supporting intralipids in chloroquine toxicity.
- What are the indications for extracorporeal membrane oxygenation (ECMO)?
- If refractory to standard supportive care and the above therapies (i.e. Escalation of epinephrine drip rate to > 3 mg/hr, presence of end-organ failure Veno-arterial ECMO has been observed to have better outcomes.
Summary
- Chloroquine and Hydroxychloroquine are being used off-label in hospitalized patients with COVID-19 infections.
- As information touting both drugs efficacy becomes widespread, there is an high-potential for toxicity from supratherapeutic ingestions, whether through self-medication, intentional, or unintentional/exploratory.
- In acute overdoses, toxicity includes hypokalemia and hypoglycemia with rapid CNS depression and cardiovascular collapse due to dysrhythmias.
- Treatment includes GI decontamination when appropriate, early intubation with high-dose epinephrine and high-dose diazepam. Refractory toxicity may be treated with VA-ECMO.
References
- World Health Organization. Coronavirus disease (COVID-19) outbreak. World Health Organization website. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Updated March 19, 2020. Accessed March 19, 2020.
- Cohen M. Trump spreads optimism for potential coronavirus drugs while public health officials cautiously wait for proof. CNN Politics Website. https://www.cnn.com/2020/04/01/politics/trump-chloroquine-drugs-coronavirus-treatment/index.html. Updated April 1, 2020. Accessed April 1, 2020.
- Waldrop T, Alsup D, McLaughlin EC. Fearing coronavirus, Arizona man dies after taking a form of chloroquine used to treat aquariums. CNN Health Website. https://www.cnn.com/2020/03/23/health/arizona-coronavirus-chloroquine-death/index.html. Updated March 25, 2020. Accessed April 1, 2020.
- Busari S, Adebayo B. Nigeria records chloroquine poisoning after Trump endorses it for coronavirus treatment. CNN World Website. https://www.cnn.com/2020/03/23/africa/chloroquine-trump-nigeria-intl/index.html. Updated March 23, 2020. Accessed April 1, 2020.
- US FDA Administration. Emergency Use Authorization. US FDA Administration Website. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#covidtherapeutics. Updated March 31, 2020. Accessed April 1, 2020.
- Wheeler M. Current Drug Shortages: Hydroxychloroquine Sulfate Tablets. American Society of Hospital Pharmacists website. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortage-Detail.aspx?id=646. Updated March 19, 2020. Accessed March 23, 2020.
- McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med. 1983;75(1A):11–18. doi:10.1016/0002-9343(83)91265-2.
- Ball DE, Tagwireyi D, Nhachi CF. Chloroquine poisoning in Zimbabwe: a toxicoepidemiological study. J Appl Toxicol. 2002;22(5):311–315. doi:10.1002/jat.864.
- Queen HF, Tapfumaneyi C, Lewis RJ. The rising incidence of serious chloroquine overdose in Harare, Zimbabwe: emergency department surveillance in the developing world. Trop Doct. 1999;29(3):139–141. doi:10.1177/004947559902900305.
- Meeran K, Jacobs MG. Chloroquine poisoning. Rapidly fatal without treatment. BMJ. 1993;307(6895):49-50. doi: 10.1136/bmj.307.6895.49.
- Riou B, Barriot P, Rimailho A, Baud FJ. Treatment of severe chloroquine poisoning. N Engl J Med. 1988;318(1):1–6. doi:10.1056/NEJM198801073180101.
- Clemessy JL, Taboulet P, Hoffman JR, et al. Treatment of acute chloroquine poisoning: a 5-year experience. Crit Care Med. 1996;24(7):1189–1195. doi:10.1097/00003246-199607000-00021.
- Wang M, Cao R, Zhang L. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020: 30, 269–271. https://doi.org/10.1038/s41422-020-0282-0.
- Yan, Y., Zou, Z., Sun, Y. et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300–302 https://doi.org/10.1038/cr.2012.165
- Barry J. Antimalarials. In: Nelson LS, Howland M, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS. eds. Goldfrank’s Toxicologic Emergencies, 11e New York, NY: McGraw-Hill; . http://accesspharmacy.mhmedical.com/content.aspx?bookid=2569§ionid=210273082. Accessed March 23, 2020.
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. [published online ahead of print, 2020 Mar 31]. medRxiv. 2020;3.22.20040758. doi: 10.1101/2020.03.22.20040758
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. [In Press, Journal Pre-proof, Available online 20 March 2020] Internaitonal Journal of Antimicrobial agents. 2020. doi.org/10.1016/j.ijantimicag.2020.105949.
- Cortegiani A, Ingoglia G, Ippolito M et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020; (PubMed 32173110) (DOI 10.1016/j.jcrc.2020.03.005).
- Study to evaluate efficacy and safety of hydroxychloroquine for treatment of pneumonia caused by 2019-nCoV (HC-nCoV). NCT04261517. https://www.clinicaltrials.gov/ct2/show/NCT04261517.
- Yao X, Ye F, Zhang M et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syn-drome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020; In Press. (PubMed 32150618) (DOI 10.1093/cid/ciaa237).
- National Health Commission (NHC) & State Administration of Traditional Chinese Medicine (Trial Version 7). Diagnosis and treatment protocol for novel coronavirus pneumonia. (http://busan. china-consulate.org/chn/zt/4/P020200310548447287942.pdf).
- Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015;122(1):110–116. doi:10.1016/j.ophtha.2014.07.018.
- Stokkermans TJ, Trichonas G. Chloroquine And Hydroxychloroquine Toxicity. NCBI Website. https://www.ncbi.nlm.nih.gov/books/NBK537086/. Updated Jun 4 2019. Accessed April 1, 2020.
- Marcucci C, Sandson NB, DeCaro MV, et al. Summary of Chloroquine and Hydroxychloroquine Drug-Drug Interactions. APSF Website. https://www.apsf.org/ddi/summary-of-chloroquine-and-hydroxychloroquine-drug-drug-interactions/. Updated March 27th, 2020. Accessed April 1, 2020.
- US FDA. ARALEN® CHLOROQUINE PHOSPHATE, USP. US FDA website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/006002s045lbl.pdf. Updated October 2018. Accessed April 1, 2020.
- McBeth PB, Missirlis PI, Brar H, Dhingra V. Novel Therapies for Myocardial Irritability following Extreme Hydroxychloroquine Toxicity. Case Rep Emerg Med. 2015;2015:692948. doi:10.1155/2015/692948.
- Gunja N, Roberts D, McCoubrie D, et al. Survival after massive hydroxychloroquine overdose. Anaesth Intensive Care. 2009;37(1):130–133. doi:10.1177/0310057X0903700112.








