Authors: Maiya Cowan, MD (EM Resident Physician, Brown University/Rhode Island Hospital) and Andrew Beck, MD (EM Attending Physician, Brown University/Rhode Island Hospital) // Reviewed by: Joshua Lowe, MD (EM Attending Physician, USAF); Marina Boushra, MD (EM-CCM, Cleveland Clinic Foundation); Brit Long, MD (@long_brit)
Case
A 72-year-old female with a past medical history of hypertension and dementia presents to the ED (emergency department) via EMS (emergency medical services) with altered mental status. The patient currently resides at a skilled nursing facility. Per staff, she has been acting progressively more tired, confused, and less interactive over the last three days. She has also had multiple episodes of urinary incontinence during this time, which is a deviation from her baseline. Today, she would not get out of bed, prompting the facility to call EMS. She had a fever of 38.7 °C (101.7 F) in the ambulance. On arrival to the ED, her blood pressure is 84/36 mmHg with a heart rate of 110 beats per minute. Septic shock is high on the differential diagnosis for this patient’s presentation. What are the most up-to-date guidelines for managing this patient?
Introduction
Sepsis and septic shock are life-threatening conditions characterized by severe systemic inflammation and organ dysfunction due to a dysregulated host response to infection.1,2 The mortality of these conditions ranges from 15-17% of those affected, killing millions of people each year.3,4 Prompt recognition and management of sepsis and septic shock are paramount for the ED clinician. The most recent surviving sepsis campaign (SSC) guidelines were released in 2021, which provided 93 recommendations for evidence-based sepsis management. This practice update will review the campaign recommendations and suggestions relevant to the ED clinician and relevant literature published since the SSC.
Screening/Diagnosis
Identifying patients with sepsis is the first step in management. The 2021 SSC no longer recommends the use of the quick sequential organ failure assessment (qSOFA) as a single screening tool for sepsis or septic shock compared to systemic inflammatory response syndrome (SIRS), national early warning score (NEWS), or modified early warning score (MEWS). This was based on studies that demonstrated qSOFA was more specific but less sensitive than its counterparts (Table 1).1
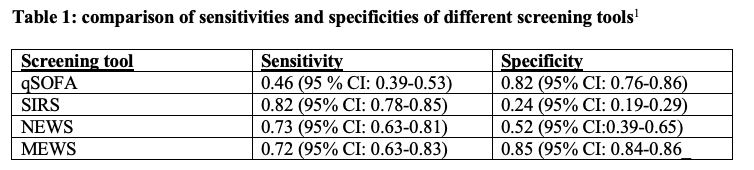
Since 2021, attempts to identify a single screening tool with optimal sensitivity and specificity to predict which patients will develop sepsis or septic shock have been ongoing. Multiple studies published in 2022 have indicated that, despite disagreement surrounding prognostic accuracy, no such screening tool so far exists.5,7 In summation, literature since the 2021 guidelines remains inconsistent in terms of the use of and application of sepsis screening scores such as qSOFA. This supports the notion that there is insufficient evidence to use qSOFA as a single screening tool in place of SIRS, NEWS, or MEWS.
In addition to clinical screening methods, many biomarkers have emerged over the years as potential adjuncts for early diagnosis of sepsis. These include C-reactive protein, procalcitonin, interleukin-6, CD64, presepsin, and sTREM-1.8 Based on multiple randomized controlled trials (RCTs), SSC 2021 guidelines suggest against using procalcitonin as a screening method to guide initiation of antibiotic therapy, as no difference in clinical outcomes was appreciated.1 Procalcitonin, an acute phase reactant that is most responsive to inflammation of bacterial origin, has been the most extensively reviewed. Current studies suggest procalcitonin levels only be interpreted in combination with the clinical context, rather than used as a single screening modality to guide therapy.8,9 Recently, monocyte distribution width (MDW) has shown promise in a large meta-analysis as a useful screening tool in the ED.10 This value reflects the heterogeneity in the size of circulating monocytes. When analyzing the receiver operating characteristic (ROC) curve of the pooled data, optimal diagnostic accuracy of MDW for sepsis prediction was seen (pooled sensitivity of 0.838 and specificity of 0.704 for Sepsis-3 criteria).10 Interestingly, another study found that the sepsis index, which divides MDW by mean monocyte volume, displayed increased specificity (0.84 vs. 0.91), positive predictive value (0.27 vs. 0.38), and positive likelihood ratio (5.08 vs. 8.52) when compared to MDW alone.11 Given that these values are generated from a complete blood count assay (CBC), which is easy and quick to obtain, these markers have the potential to be adopted into future clinical practice, though validating studies are still pending.
Respiratory support
Acute hypoxemic respiratory failure can be a sequela of sepsis. It typically arises from either pneumonia or extra-pulmonary infections that may lead to acute respiratory distress syndrome (ARDS). For hypoxic, non-hypercarbic patients that require advanced interventions, SSC 2021 now suggests a trial of high flow nasal cannula (HFNC) as the first step over noninvasive ventilation (NIV). The rationale is that HFNC can provide some positive pressure, while avoiding certain complications of NIV, such as gastric insufflation, aspiration, and higher tidal volumes.1 This recommendation was primarily based on a single large RCT that showed improved ninety-day survival with HFNC when compared with NIV.12 Since then, evidence continues to be limited concerning the initiation of HFNC vs. NIV for sepsis and hypoxia in the ED. However, more recent data supports the use of HFNC in this subset, notably when weaning from mechanical ventilation or preventing reintubation.13,14
Fluids
Fluid resuscitation is a mainstay of sepsis therapy, as the condition is commonly associated with both absolute and relative hypovolemia. This is primarily a result of increased vascular permeability and decreased vascular tone, leading to vasodilation.15 Two changes were elucidated in the 2021 SSC guidelines for fluid resuscitation. The first was the suggestion to use balanced crystalloid fluids, such as lactated ringers or plasma-lyte, instead of normal saline. The second was the downgrade of using a 30 cc/kg bolus within the first three hours from a strong, low quality of evidence to a weak, low quality of evidence suggestion.1
The rationale for the shift away from normal saline in 2021 derived from multiple trials, including the Isotonic Solutions and Major Adverse Renal Events Trial (SMART) and Saline Against Lactated Ringer’s or Plasma-Lyte in the Emergency Department (SALT ED).16,17 These studies found that high volumes of normal saline are more commonly associated with hyperchloremic metabolic acidosis and acute kidney injury (AKI) when compared with balanced crystalloids. However, more recently, the Plasma-Lyte 148 versus Saline (PLUS) and Balanced Solution versus Saline in Intensive Care Study (BasICS) trials have shown no difference in mortality or AKI when comparing balanced crystalloids to saline in critically ill patients.18,19 Since the 2021 SSC recommendations, many papers have shown no meaningful difference in mortality based on fluid choice. It is not clear currently which fluid, if any, is the optimal choice for volume expansion in patients with sepsis. However, fluid resuscitation remains a cornerstone of sepsis care and should not be neglected in the ED.
Additionally, the recommendation to use a 30 cc/kg bolus was downgraded. This is due to a lack of controlled studies comparing different fluid volumes against one another. Even so, the use of a 30 cc/kg bolus has been adopted into routine clinical practice. The current suggestion for septic patients with hypoperfusion is to administer a 30 cc/kg ideal body weight bolus within the first three hours and then tailor additional fluid resuscitation to individualized hemodynamic measurements.1 Of note, the 30 cc/kg strategy should only be employed in those patients exhibiting signs of hypoperfusion. There are circumstances where such an approach may require consideration of additional comorbidities such as hypervolemic states, cardiac dysfunction, pulmonary hypertension, etc. Studies in the last three years have shown that septic patients with signs of hypoperfusion benefit from the initial 30 cc/kg bolus regardless of comorbidities such as congestive heart failure (CHF), cirrhosis, or end-stage renal disease (ESRD).20-22 However, there may be patients in which such conditions may warrant a more conservative approach for continued resuscitation. The more recent CLASSIC and CLOVERS trials have shown no difference in mortality between a conservative and liberal fluid resuscitation strategy after initial resuscitation.23,24 The CLOVERS trial also noted this lack of effect in the subgroups of participants with CHF and ESRD.24 While 30 cc/kg remains the standard for initial resuscitation in the ED, the downgrade of this recommendation sheds light on the lack of contextual data to support this volume. Additionally, the emergency physician should consider all fluid resuscitation strategies in the context of presenting illness and comorbidities, as the risk of potential harm does exist.
Observational studies support the use of dynamic measures of volume status to guide continued fluid resuscitation. This includes passive leg raise, pulse pressure variation, systolic volume variation, and inferior vena cava (IVC) diameter variation.25,26 These methods can help delineate between patients who are fluid-responsive and those in whom additional volume may be harmful.26 While large RCTs are needed to confirm this observation, these methods are easily employable, even in low-resource settings. Furthermore, since the 2021 guidelines, multiple RCTs have shown that utilizing IVC respiratory variation, assessed by bedside ultrasound, as a guide to fluid resuscitation can result in less fluid administered. However, there has been no proven mortality benefit.27,28
Additionally, guiding fluid resuscitation based on capillary refill time (CRT) is a new SSC guideline suggestion.1 This was based on the ANDROMEDA SHOCK trial, which demonstrated that guiding resuscitation to normalization of CRT compared to decreasing lactate levels resulted in significantly less organ dysfunction as assessed by SOFA score. Despite these findings, no difference in mortality was observed.29 More recently, a meta-analysis of ten studies indicated there may be a poor inverse correlation between CRT and MAP.30 Though empirically lacking, CRT has utility as a non-invasive and no-cost method to guide management, especially in resource-limited settings. Trending lactate as a guide to fluid resuscitation is also only weakly suggested when resources permit.1
Vasopressors
The use of vasopressor support in persistently hypotensive patients with septic shock is widely accepted. Current guidelines recommend titrating vasopressors to a mean arterial pressure (MAP) of 65 mmHg in initial resuscitation.1 This is preferred over higher MAP goals, as data indicates that there is no difference in mortality when utilizing higher MAP targets.31 A new suggestion in the 2021 SSC is to start pressors peripherally without delaying initiation until central access is obtained. The rationale is that vasopressors can be safely given through a peripheral IV, at or more proximal to the antecubital fossa, for six hours before increasing any known morbidity or mortality risk. Therefore, delayed initiation of vasopressors poses a greater risk for harm than potential extravasation or local tissue ischemia from peripheral administration.1
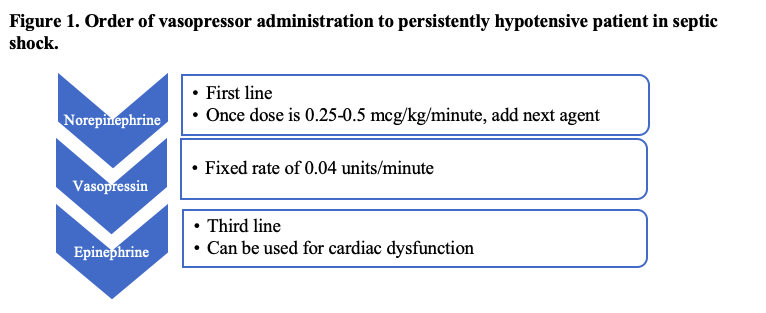
The initial vasopressor of choice for septic shock is norepinephrine, as early administration has been shown to increase survival in these patients.32,33 Once the dose of norepinephrine is between 0.25-0.5 mcg/kg/minute, vasopressin should be added at a fixed rate of 0.04 units/minute.1 Attempts to reduce the dose of norepinephrine should be trialed at this time to avoid adrenergic overload.34 If a MAP of 65 mmHg is still not achieved, epinephrine should be added as a third agent (Figure 1). For patients with septic shock and cardiac dysfunction that are persistently hypotensive, it is appropriate to use norepinephrine and dobutamine or epinephrine alone.1
Vasopressors are a widely accepted therapy for persistently hypotensive patients with septic shock. The 2021 suggestion to start vasopressors peripherally illustrates the importance of prompt initiation in the ED when necessary. Evidence continues to support norepinephrine, vasopressin, and epinephrine as the order of vasopressor initiation, respectively, titrated to MAP of 65 mmHg.
Antibiotics
Antibiotic delivery is one of the most effective interventions to decrease mortality in patients with sepsis and septic shock. There has been much discussion about the appropriate timing of administration of initial antibiotics while attempting to minimize exposure to unnecessary antibiotics. Current SSC guidelines recommend the time from presentation to antibiotic administration as one hour. This is a strong recommendation for both patients presenting in septic shock and those with a “high likelihood” of having sepsis.1 However, given that the benefit for patients with sepsis who are not in shock is not as apparent if the diagnosis is unclear, antibiotics can be delayed for up to three hours to allow further infectious workup and avoid inappropriate antibiotic delivery.1,35
The specific antibiotic agent should be chosen based on the anticipated site of infection. The Infectious Diseases Society of America (IDSA) guidelines provide the most up-to-date guidance on appropriate agent choice.36 Regardless of site, all patients with suspected sepsis or septic shock should receive broad-spectrum antibiotics while cultures are pending.1 The addition of other agents is tailored on a case-by-case basis (Figure 2). If the patient is at high risk for infection with multi-drug resistant (MDR) organisms, then two agents with gram-negative coverage should be administered (Table 2).1 The rationale behind this suggestion includes theoretical benefits such as the potential for synergistic activity, more rapid pathogen clearance, and preventing the development of antimicrobial resistance.36 However, the literature has failed to prove that the double coverage has any effect on clinical outcomes. 36,37

The decision regarding whether to add methicillin-resistant staph aureus (MRSA) coverage is based on specific risk factors for MRSA (Table 3).1 Similarly, if a patient is at high risk for fungal infection, then empiric antifungals should be administered as well (Table 4).1 There is no recommendation for the use of antiviral medications. Once susceptibilities are determined, antibiotic therapy should be narrowed appropriately.36 The ideal antibiotic carries an acceptable side effect and drug-interaction profile, with the narrowest spectrum to prevent unnecessary antibiotic resistance.

Antibiotics are a critical part of initial sepsis management in the ED. They should be given promptly and tailored based on anticipated site of infection and individual patient risk factors.
Adjunctive therapy (steroids, thiamine, and vitamin C)
Multiple adjunctive therapies have been studied for their utility in the management of sepsis. The current SSC guidelines suggest the use of stress dose IV corticosteroids (200 mg hydrocortisone per day) for adults with septic shock and an ongoing vasopressor requirement for at least four hours after initiation.1 This is a change from the 2016 SSC guidelines which suggested against corticosteroids.38 Steroids work by both regulating the immune response and enhancing cardiovascular response to catecholamines.39 Furthermore, SSC 2021 suggests against the use of vitamin C.1
The only adjunctive therapy proven to have benefit is IV corticosteroids, but other therapies have also been explored.1 It was proposed that sepsis may contribute to vitamin deficiencies, paving the way for thiamine as a potential adjunct. Additionally, vitamin C plays a role in inflammation.40 However, despite the anti-inflammatory properties of vitamin C, pooled data from seven RCTs shows increased mortality compared to placebo.41 Similarly, studies have analyzed vitamin C, thiamine, and hydrocortisone for a synergistic effect when given together in patients with sepsis. These studies found no difference in mortality when compared to hydrocortisone alone.40,42
Studies since the 2021 SSC guidelines continue to support the use of IV corticosteroids for patients with septic shock and an ongoing vasopressor requirement. More literature has been published that found no clinically significant benefit for other adjunctive therapies, such as vitamin C and thiamine. ED clinicians should continue to utilize stress-dose steroids when indicated.
Disposition
The SSC guidelines suggest that patients with septic shock or critical illness be moved to the intensive care unit (ICU) within six hours of presentation to the ED. This recommendation is largely based on observational data which shows that timely admission of critically ill patients to an ICU environment may result in better patient outcomes and decreased mortality.1
Conclusion
The recommendations for the management of sepsis in the ED continue to be dynamic, and research regarding optimal sepsis management is ongoing. The SSC released the latest evidence-based recommendations in 2021. Since then, many of the guidelines have been adopted into routine clinical practice. New data has emerged that both support and refute some of these guidelines. This article outlined the most up-to-date recommendations and literature for the management of patients with sepsis and septic shock related to the ED clinician.
Pearls and pitfalls
- qSOFA should no longer be used as a single screening tool for sepsis.
- A trial of HFNC is suggested over NIV for hypoxemic, non-hypercarbic patients with sepsis.
- In patients presenting in septic shock, it is suggested that 30 cc/kg of balanced crystalloid should be given within the first three hours of ED presentation. Dynamic measures of volume status should be used to guide additional fluid resuscitation.
- Antibiotic therapy should include a broad-spectrum agent with gram-negative coverage. Additional agents should be based on individual patient risk factors.
- IV steroids should be used as an adjunct for patients with a persistent vasopressor requirement.
References
- Evans L, Rhodes A, Alhazzani W, et al.Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247.
- Piccioni A, Saviano A, Cicchinelli S, et al. Proadrenomedullin in Sepsis and Septic Shock: A Role in the Emergency Department. Medicina. 2021;57(9):920.
- Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272.
- Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318(13):1241–1249.
- Wang C, Xu R, Yuerong Z, et al. A comparison of qSOFA, SIRS and NEWS in predicting the accuracy of mortality in patients with suspected sepsis: A meta-analysis. PLOS ONE. 2022;17(4):e0266755.
- Fullerton JN, Price CL, Silvery NE, et al. Is the Modified Early Warning Score (MEWS) superior to clinician judgement in detecting critical illness in the pre-hospital environment?. 2012;83(5):557-562
- Sparks R, Harada A, Chavada R, et al.Comparison of different sepsis scoring systems and pathways: qSOFA, SIRS, Shapiro criteria and CEC SEPSIS KILLS pathway in bacteraemic and non-bacteraemic patients presenting to the emergency department. BMC Infect Dis. 2022;22:76.
- Hung S, Lan H, Han S, et al. Current Evidence and Limitation of Biomarkers for Detecting Sepsis and Systemic Infection. 2020;8:494.
- Velissaris D, Zareifopoulos N, Lagadinou M, et al. Procalcitonin and sepsis in the Emergency Department: an update. Rev. Med. Pharmacol. 2021;25:466-479.
- Agnello L, Vidali M, Lo Sasso B, et al. Monocyte distribution width (MDW) as a screening tool for early detecting sepsis: a systematic review and meta-analysis. Clinical Chemistry and Laboratory Medicine (CCLM). 2022;60(5):786-792.
- Agnello L, Iacona A, Maestri S, et al. Independent Validation of Sepsis Index for Sepsis Screening in the Emergency Department. Diagnostics. 2021;11:1292.
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196.
- Xuan L, Ma J, Tao J, et al. Comparative study of high flow nasal catheter device and noninvasive positive pressure ventilation for sequential treatment in sepsis patients after weaning from mechanical ventilation in intensive care unit. Palliat. Med.2021;10:6270–6278.
- Tongyoo S, Tantibundit P, Daorattanachai K, et al. High-flow nasal oxygen cannula vs. noninvasive mechanical ventilation to prevent reintubation in sepsis: A randomized controlled trial. Intensive Care.2021;11:135.
- Gavelli F, Castello LM, Avanzi GC. Management of sepsis and septic shock in the emergency department. Intern Emerg Med. 2021;16:1649–1661.
- Semler MW, Self WH, Wanderer JP, et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med. 2018;378(9):829-839.
- Self WH, Semler MW, Wanderer JP, et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. N Engl J Med. 2018;378(9):819-828.
- Finfer S, Micallef S, Hammond N, et al. Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults. N Engl J Med. 2022;386(9):815-826.
- Zampieri FG, Machado FR, Biondi RS, et al. Effect of Intravenous Fluid Treatment with a Balanced Solution vs 0.9% Saline Solution on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. 2021;326(9):818–829.
- Taenzer AH, Patel SJ, Allen TL, et al. Improvement in Mortality with Early Fluid Bolus in Sepsis Patients with a History of Congestive Heart Failure. Mayo Clin. Proc. 2020;4(5):537-541.
- Acharya R, Patel A, Schultz E, et al. Fluid Resuscitation and Outcomes in Heart Failure Patients with Severe Sepsis or Septic Shock: a Retrospective Case-control Study. PLoS One. 2021;16(8):e0256368
- Khan RA, Khan NA, Bauer SR, et al. Association Between Volume of Fluid Resuscitation and Intubation in High-Risk Patients with Sepsis, Heart Failure, End-stage Renal Disease, and Cirrhosis. 2020;157(2):286-292.
- Meyhoff TS, Hjortrup PB, Wetterslev J, et al. Restriction of Intravenous Fluid in ICU Patients with Septic Shock. N Engl J Med. 2022;386:2459-2470.
- Shapiro NI, Douglas IS, Brower RG, et al. Early Restrictive or Liberal Fluid Management for Sepsis-induced Hypotension. N Engl J Med. 2023;388:499-510.
- Dubin A, Loudet C, Vanina S, et al. Characteristics of resuscitation, and association between use of dynamic tests of fluid responsiveness and outcomes in septic patients: results of a multicenter prospective cohort study in Argentina. Intensive Care. 2020;10:40.
- Bakker J, Kattan E, Annane D, et al.Current practice and evolving concepts in septic shock resuscitation. Intensive Care Med. 2022;48:148–163.
- Musikatavorn K, Plitawanon P, Lumlertgul S, et al. Randomized Controlled Trial of Ultrasound-guided Fluid Resuscitation of Sepsis-Induced Hypoperfusion and Septic Shock. West J Emerg Med. 2021;22(2).
- Zhuang Y, Dai L, Cheng L, et al. Inferior vena cava diameter combined with lung ultrasound B-line score to guide fluid resuscitation in patients with septic shock. Zhonghua wei zhong bing ji jiu yi xue. 2020;32(11):1356-1360.
- Hernandez G, Ospina-Tascon GA, Damiani LP, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA. 2019;321(7):654–664.
- Putowski Z, Gołdyn M, Pluta MP, et al. Correlation Between Mean Arterial Pressure and Capillary Refill Time in Patients with Septic Shock: A Systematic Review and Meta-analysis. J Intensive Care Med. 2023. Accessed June 10, 2023. https://journals.sagepub.com/doi/10.1177/08850666231168038
- Lamontagne F, Richards-Belle A, Thomas K, et al. Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients with Vasodilatory Hypotension: A Randomized Clinical Trial. 2020;323(10):938–949.
- Jouffroy R, Hajjar A, Gilbert B, et al.Prehospital norepinephrine administration reduces 30-day mortality among septic shock patients. BMC Infect Dis. 2022;22:345
- Alshahrani MS and Alatigue R. Association Between Early Administration of Norepinephrine in Septic Shock and Survival. Open Access Emerg Med. 2021;13:143-150.
- Guarino M, Perna B, Cesaro AE, et al. 2023 Update on Sepsis and Septic Shock in Adult Patients: Management in the Emergency Department. J Clin Med. 2023;12(9):3188.
- Im Y, Kang D, Ko RE, et al.Time-to-antibiotics and clinical outcomes in patients with sepsis and septic shock: a prospective nationwide multicenter cohort study. Crit Care. 2022;26:19.
- Strich JR, Heil EL, Masur H. Considerations for Empiric Antimicrobial Therapy in Sepsis and Septic Shock in an Era of Antimicrobial Resistance. J Infect Dis. 2020;222(2):S110-S131.
- Niederman MS, Baron RM, Boudama L, et al. Initial Antimicrobial Management of Sepsis. Crit Care. 2021;25:307
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304–377
- Andrés R, Noriega L, Montes Argumedo J, et al. Utility of Corticoids in Septic Shock. Aditum Journal of Clinical and Biochemical Research. 2022;4(1) http;//doi.org/01.2022/1.1067.
- Moskowitz A, Huang DT, Hou PC, et al. Effect of Ascorbic Acid, Corticosteroids, and Thiamine on Organ Injury in Septic Shock: The ACTS Randomized Clinical Trial. JAMA. 2020;324(7):642-650.
- Lamontagne F, Masse M, Menard J, et al. Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit. New Engl J Med. 2022;386(25):2387-2398.
- Fujii T, Luethi N, Young PJ, et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial. 2020;323(5):423–431.









1 thought on “Sepsis Updates Relevant to the Emergency Physician”
With whatever respect the authors were due, qSOFA was never anything other than an ego trip for the authors. It was obvious from the beginning that it was a wrong turn. If it’s not broken, why fix it? SIRS, Sepsis, Severe Sepsis, and Septic Shock were clearly descriptive the way that Rivers et al., described them. The rest has been frankly, a mess, just something to confuse students and cause controversy which has at best detracted from patient care standards.