Authors: Sean Trostel, MD (EM Resident Physician, Carolinas Medical Center, Charlotte, NC); Kathryn T Kopec, DO (@KopecToxEM, EM Attending Physician, Medical Toxicologist, Carolinas Medical Center, Charlotte, NC) // Reviewed by: James Dazhe Cao, MD (@JamesCaoMD, Associate Professor of EM, Medical Toxicology, UT Southwestern Medical Center, Dallas, TX); Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)

Please see Part 1 of this series for guidance on the management of the crotalid envenomation.
Case, continued:
A 2-year-old boy was admitted to your hospital in stable condition after a copperhead snake bite to the right lower leg. He was administered 10 vials of Crotalidae equine immune F(ab’)2 (ANAVIP®) at the outside hospital before transfer to your referral center.
Approximately 12 hours following F(ab’)2 antivenom administration, the child is noted to have progressive swelling, firmness, tension, and pain of the right calf. Your hospital pharmacy stocks only crotalidae ovine polyvalent immune F(ab) (CroFab®) antivenom.
Questions:
- What antivenoms are available to treat crotalid envenomations?
- What are the major similarities and differences between available crotalid antivenoms?
- What is the dosing schedule for each antivenom?
Background
Crotalidae ovine polyvalent immune F(ab) (CroFab®) is the most widely available crotalid antivenom in the United States and is approved for severe crotalid envenomation with severe or progressive localized symptoms, systemic toxicity, and signs of hematologic toxicity on labs. However, in 2015 crotalidae equine immune F(ab’)2 (ANAVIP®) was approved by the FDA for rattlesnake envenomations, and distribution of this antivenom began in 2018. In April 2021, after a post-hoc analysis of the original phase 3 study suggested non-inferiority of the F(ab’)2 antivenin against copperhead envenomation, the FDA expanded approval of this antivenom to include copperhead and cottonmouth envenomations.1,2
Discussion: F(ab) vs. F(ab’)2 Antivenoms
While both antivenoms are approved to treat all crotalid envenomations in the United States, the commercially available F(ab) and F(ab’)2 antivenoms have several noteworthy differences:
-
- The F(ab) antivenom is produced from a sheep-derived (ovine) antibody, while F(ab’)2 is horse-derived (equine).
- F(ab) is smaller, consisting of a single antigen binding site, while F(ab’)2 consists of two antigen-binding sites linked together by an antibody “hinge” region. This size difference may lead to differences in the pharmacokinetic profiles of the two antibodies.
- Plasma half-life of the F(ab’)2 antivenom is significantly longer at 5.5 days, compared to 12-23 hours of the F(ab) antivenom, reflecting its tendency to remain in the intravascular compartment.3,4
- The smaller size of F(ab) antivenom may promote more rapid tissue penetration as compared to F(ab’)2.5
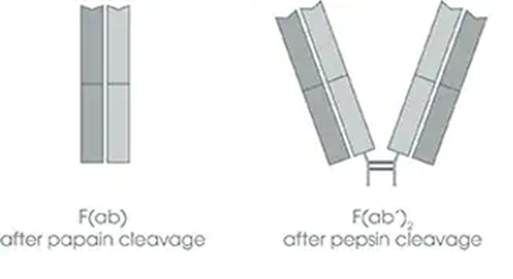
Figure 1. F(ab) versus F(ab)2 structural differences. (Source: https://www.abcam.com/secondary-antibodies/advantages-of-immunoglobulin-fab-and-fab2-fragments)
- F(ab) antivenom is produced with venoms from four North American pit vipers, including the western diamondback rattlesnake (Crotalus atrox), eastern diamondback rattlesnake (Crotalus adamanteus), Mojave rattlesnake (Crotalus scutulatus), and cottonmouth (Agkistrodon piscivorus) venoms. F(ab’)2 antivenom is produced with venoms from two South and Central American pit vipers: the South American rattlesnake (Crotalus durissus) and terciopelo (Bothrops asper).
Table 1: Comparison of F(ab) and F(ab’)2 antivenoms.3,4,6,7,8
[table id=13 /]
In a phase 3 trial comparing F(ab) to F(ab’)2 antivenom, the F(ab’)2 antivenom was associated with significantly lower rates of recurrent or late thrombocytopenia than F(ab) antivenom, possibly attributable to the F(ab’)2 antivenom’s longer plasma half-life.9 This effect may be especially prominent in the setting of rattlesnake envenomation, where coagulopathic effects are more prominent.
The addition of Agkistrodon venoms in the production of the F(ab) antivenom may also provide better specificity and efficacy against these species. As we commonly see in practice, differences exist in the clinical and toxic effects of envenomation by snakes even within the same genus. For example, Mojave rattlesnake envenomation may produce neurotoxic effects not seen in envenomations by most other rattlesnake species. This suggests that venom profiles are not identical between species, and thus different antibodies may be more or less efficacious against specific venoms.10,11
Expansion of the new F(ab’)2 antivenom’s indications to include envenomation by the Agkistrodon genus (copperhead/cottonmouth) was based on a single abstract describing a post-hoc non-inferiority analysis of 21 patients from the original F(ab’)2 antivenom phase 3 trial. This analysis suggested that the F(ab’)2 antivenom was noninferior to F(ab) antivenom in multiple measures. However, due to the limited sample size, confidence intervals were wide for all of these measures, and only 2 of 3 examined measures met the predetermined non-inferiority assumptions.1,2 More research is needed to determine whether a difference truly exists in efficacy of these antivenoms against local tissue effects which predominate after Agkistrodon envenomation.
Adverse Effects:
- F(ab) (CroFab®)4
- Less than 5% of people will experience urticaria, rash, nausea, and pruritus
- Anaphylaxis & anaphylactoid reactions can occur
- Recurrent coagulopathy is common and is characterized by decreased fibrinogen, decreased platelets, and elevated prothrombin time
- F(ab’)2 (ANAVIP®)3
- Less than 2% of people experience pruritus, nausea, rash, arthralgia, peripheral edema, myalgia, and headache
- Anaphylactic reactions are possible
Case Follow-up:
Secondary to the recurrent, progressive swelling and pain of the right calf, 4 vials of F(ab) antivenom were administered in addition to the initial 10 vials of F(ab’)2 antivenom. Following this second dose, the firmness and swelling of the leg improved, and the patient had an otherwise uneventful hospital course. Labs including platelets and fibrinogen remained normal, and the patient was discharged on hospital day 4. On follow-up 1 week later, the patient was doing well, ambulating and playing without significant discomfort.
Clinical Pearls:
- Most snake envenomations in the United States are caused by pit vipers (crotalidae) and are characterized by local tissue injury and coagulopathic effects.
- Copperhead and cottonmouth (Agkistrodon) envenomations are generally characterized by more prominent local tissue effects, while rattlesnake (Crotalus) envenomations often cause early, late, and recurrent coagulopathy in addition to local tissue effects.
- F(ab) (CroFab®) and F(ab’)2 (ANAVIP®) crotalid antivenoms are FDA approved and indicated for severe crotalid envenomation with severe or progressive localized symptoms, systemic symptoms, and/or signs of coagulopathy on labs.
- Research suggests that F(Ab’)2 antivenom, compared against F(ab) antivenom, may be associated with lower rates of late and recurrent coagulopathy.9
- More research is needed to determine whether significant differences exist between antivenoms in efficacy against local tissue injury or efficacy against specific species of snake.
References:
- Ubani C, DeGelorm A, Sollee D. Poison Control: The F(ab2)ulous Expanded Indication of Anavip | FCEP. Accessed May 7, 2022. https://fcep.org/poison-control-the-fab2ulous-expanded-indication-of-anavip/
- Gerardo CJ, Keyler DE, Rapp-Olsson AM, Schwarz J, Dart RC. 72 Post Hoc Analysis of the RCT Comparing F(ab’)2to Fab Antivenom: Control of Venom-induced Tissue Injury in Copperhead Snakebite Patients. Ann Emerg Med. 2020;76(4):S29. doi:10.1016/j.annemergmed.2020.09.082
- Full prescribing information. ANAVIP. Crotalidae Immune F(ab’)2 (Equine). Published online April 2021. Accessed May 7, 2022. https://www.fda.gov/media/92139/download
- Full prescribing information. CROFAB. Crotalidae polyvalent immune fab (ovine). Published online January 2018. Accessed August 17, 2023. https://www.fda.gov/media/74683/download
- Li Z, Krippendorff BF, Sharma S, Walz AC, Lavé T, Shah DK. Influence of molecular size on tissue distribution of antibody fragments. mAbs. 2016;8(1):113-119. doi:10.1080/19420862.2015.1111497
- Antivenom administration North American Crotalinae snakebites – UpToDate. Accessed May 7, 2022. https://www.uptodate.com/contents/image/print?imageKey=EM%2F122011&topicKey=EM%2F8365&source=see_link
- Blue Ridge Poison Center, University of Virginia Health. Battle of the Antivenoms: CroFab vs ANAVIP. ToxTalks. Published online November 2020. Accessed May 7, 2022. https://med.virginia.edu/toxicology/wp-content/uploads/sites/268/2020/11/Nov20-Crofab-Anavip.pdf
- Lavonas EJ, Ruha AM, Banner W. et al. Unified Treatment Algorithm for the management of crotalid snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emerg Med. 2011;3(11):2.
- Bush SP, Ruha AM, Seifert SA, et al. Comparison of F(ab’)2 versus Fab antivenom for pit viper envenomation: A prospective, blinded, multicenter, randomized clinical trial. Clin Toxicol Phila Pa. 2015;53(1):37-45. doi:10.3109/15563650.2014.974263
- Buchanan JT, Thurman J. Crotalidae Envenomation. In: StatPearls. StatPearls Publishing; 2022. Accessed May 17, 2022. http://www.ncbi.nlm.nih.gov/books/NBK551615/
- Kanaan NC, Ray J, Stewart M, et al. Wilderness Medical Society Practice Guidelines for the Treatment of Pit viper Envenomations in the United States and Canada. Wilderness Environ Med. 2015;26(4):472-487. doi:10.1016/j.wem.2015.05.007








