Authors: Luke Wohlford, MD, MPH (@retinueofblue, EM Resident, University of Vermont Medical Center); Neil Krulewitz, DO (EM Attending, University of Vermont Medical Center); Ahmed Harhash, MD (Heart Failure Cardiologist, University of Vermont Medical Center); Skyler Lentz, MD (@SkylerLentz, EM and Critical Care Attending, University of Vermont Medical Center)
Case
A 55-year-old man with a history of heart failure with a preserved ejection fraction presents at the request of their cardiologist with weight gain, dyspnea and “high pressures on my pulmonary artery monitor.” His cardiologist has told him his CardioMEMSTM device is showing a heart failure exacerbation. He has been taking increased doses of torsemide without improvement in his symptoms and was directed to the ED for inpatient therapy. He has crackles on his lung exam, symmetric lower extremity edema and JVD. His workup reveals an elevated NT-pro BNP and evidence of pulmonary edema on a chest x-ray. As you order a dose of intravenous diuretic, you wonder what device they have and what information it can give.
Background
The detection of acute heart failure classically includes typical heart failure signs and symptoms such as weight gain, edema, and dyspnea. Now, a remote outpatient pulmonary arterial pressure monitor and other heart failure detection devices (Table 1) are available that you may begin to see in your ED. The goal of these devices is to detect a heart failure decompensation early and to reduce hospital admissions. The CardioMEMSTM by Abbott is one commercial device currently available for remote monitoring of patients with heart failure with a preserved ejection fraction (HFpEF) and heart failure with a reduced ejection fraction (HFrEF).1 Like other implantable devices, such as pacemakers and automated implantable cardioverter defibrillators (AICDs), they can be interrogated for valuable information by the patient’s cardiology team when the patient presents to the ED. Other devices assessing for heart failure include HeartLogic and OptivolTM which measure chest impedance as an indicator of pulmonary and thoracic fluid.2-4 Chest impedance can be measured by implanted cardioverter-defibrillators (ICDs) and implanted cardiac resynchronization defibrillators (CRT-D) inserted for other indications. HeartLogic further measures accelerometer-based first and third heart sounds, intrathoracic impedance, respiration rate, the ratio of respiration rate to tidal volume, night heart rate, and patient activity.2 These implantable devices offer physiological parameters to direct therapies and reduce hospitalizations.5 In the ED setting, this may be seen when the outpatient provider sends a patient to the ED for treatment of heart failure or when the patient presents themselves with cardiac complaints.

Physiology and Device Placement
In heart failure, the left and/or right ventricular fillings pressure are elevated and increase during exacerbation. An implantable device measuring the pulmonary artery pressure or thoracic impedance may detect this process early, allowing the clinician to intervene. OptivolTM, measures thoracic impedance through an ICD or CRT-D device. Once the thoracic impedance passes a threshold, suggesting increased pulmonary and thoracic fluid, the clinician may be alerted to intervene.4 The HeartLogic sensor can be embedded into CRT-D devices to assess for heart failure exacerbation using thoracic impedance, respiratory rate, tidal volume, heart rate and patient activity.2 Emergency clinicians may begin to see thoracic impedance readings when interrogating these devices for other reasons (e.g. arrhythmia). Though these devices are convenient because the technology may be implanted with a necessary device (e.g. ICD, CRT-D) in patients with heart failure, it does not have the same level of supporting evidence as the CardioMEMSTM device.
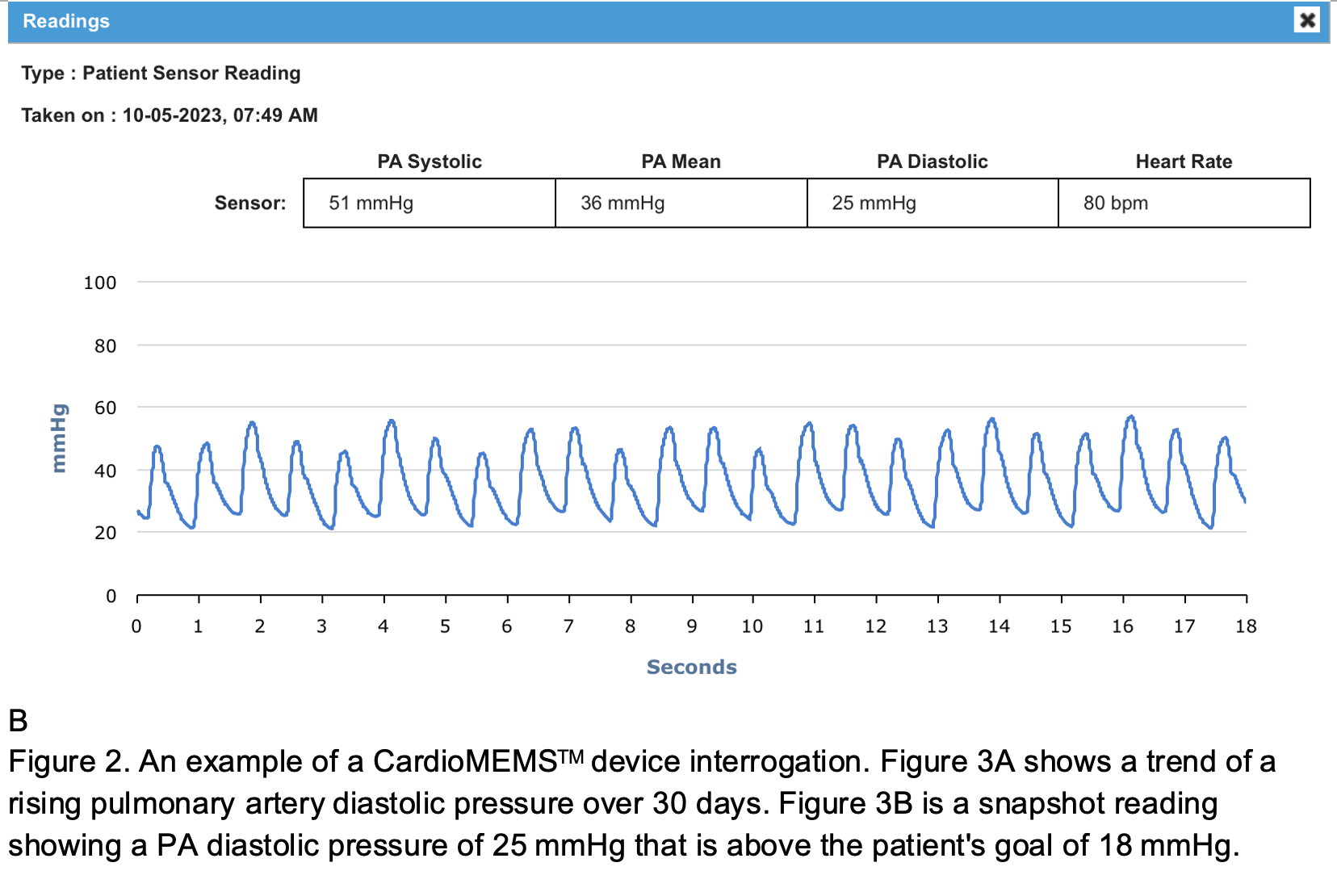
The CardioMEMSTM by Abbott gives the clinician the ability to remotely monitor their patients’ pulmonary arterial pressure using a web-based interface. Much like a traditional pulmonary arterial catheter, this device enables the heart failure specialist to obtain real-time measurements of pulmonary artery pressure. A rising diastolic pressure suggests elevation in the left atrial pressure and left ventricular filling pressure which is often an early physiologic component of heart failure exacerbations. Once a worrisome trend of a rising PA diastolic pressure is identified (Figure 2), providers can intervene with outpatient therapy such as increased diuretic dosing or adjustment of antihypertensive regimen. The PA diastolic target can be specified by the cardiologist. For instance, a PA diastolic pressure > 18 mmHg (normal 8-12 mm Hg) may alert the clinician to intervene.6
Major challenges implementing CardioMEMSTM in heart failure include the cost and invasive placement of the device.5 One study found that the cost of the device itself plus its insertion totals nearly $20,000, with additional costs to patients for monitoring and follow-up in cardiology clinic.7 This same study estimates a hospitalization for heart failure exacerbation to be nearly $20,000, indicating that the intervention could be cost effective especially over multiple years if able to avert potential exacerbations.
The approach for placing the device is akin to a right heart catheterization, with a sheath inserted in a central vein. Access is gained to the pulmonary circulation following the floating of a balloon past the tricuspid valve, and is ideally implanted in a left posterior pulmonary artery between 7 and 15mm in diameter.8 The device itself is 2 x 2.5 x 15 mm (Figure 1), with bilateral nitinol loops each 10mm in diameter to facilitate securement in the pulmonary artery.9Fortunately, this device is considered to be safe for patients undergoing MRI imaging by the FDA.10 This procedure has well-described rare complications including infection, blood loss, allergic reaction, arrhythmia, pneumothorax if approaching via the internal jugular vein, embolization of the device, and pulmonary artery perforation or dissection.10



Evidence
For nearly a decade, the only randomized controlled trial with insight into the effectiveness of CardioMEMSTM was the 2011 CHAMPION trial.12-13 This United States study demonstrated a reduction in HF exacerbation admissions in those with NYHA class III HF symptoms and recent HF hospitalization who were monitored with a CardioMEMSTMdevice. The device group experienced 39% fewer HF hospitalizations over the study period. A study using the CHAMPION data reported a cost‐effectiveness ratio of $44,832 per quality‐adjusted life year.7 Since the CHAMPION study, several other trials this decade have provided additional evidence supporting the efficacy and cost-effectiveness of the device. The MEMS-HF study based in Europe also reported benefit in the CardioMEMSTM group, with 62% fewer HF hospitalizations, or nearly one fewer admission per year in the treatment group.14 MONITOR-HF, set in the Netherlands, found a less robust but still significant reduction in HF hospitalizations (0.3 fewer admissions per patient per year), and improved reported quality of life in the treatment group.15 The final analysis of the GUIDE-HF study in the United States and Canada did not report a significant reduction in HF hospitalizations, but was likely affected by COVID-19 as a pre-COVID-19 analysis did show a reduction in HF hospitalizations similar to MONITOR-HF.16 Future implants such as Cordella use newer technology to simplify data transmission and incorporate important data such as BP, weight, etc. This newer device has already completed its first FDA-approved investigational device exemption study (IDE) that will soon be published.17
Conclusion
Emergency clinicians will begin to see heart failure patients with various implantable monitors and should understand the underlying physiology to optimize clinical use. Current evidence supports the cost-effectiveness of the CardioMEMSTM device. It is likely that this device and future devices will become more common in patients presenting to the ED.
Case Conclusion
Your patient is given an IV dose of furosemide and improves after diuresis over the next few hours in the emergency department. The heart failure cardiologist comes down to discuss the CardioMEMSTM data with you and the patient. The patient had an increasing PA diastolic pressure on the device report over the past week. The cardiologist recommends adding spironolactone and outpatient follow-up in several days. With hospitalization averted by early recognition of elevated PA pressures, the patient is grateful for the chance to spend more time at home with their dogs.
Disclaimer: No author has received funds from industry or any device company.
References
1. Abbott. CardioMEMS HF System for pulmonary artery pressure monitoring. Accessed June 23, 2023 from https://www.cardiovascular.abbott/us/en/hcp/products/heart-failure/pulmonary-pressure-monitors/cardiomems/about.html.
2. Gardner RS, Singh JP, Stancak B, Nair DG, Cao M, Schulze C, Thakur PH, An Q, Wehrenberg S, Hammill EF, Zhang Y. HeartLogic multisensor algorithm identifies patients during periods of significantly increased risk of heart failure events: results from the MultiSENSE study. Circulation: Heart Failure. 2018 Jul;11(7):e004669.
3. Capucci A, Santini L, Favale S, Pecora D, Petracci B, Calò L, Molon G, Cipolletta L, Bianchi V, Schirripa V, Santobuono VE. Preliminary experience with the multisensor HeartLogic algorithm for heart failure monitoring: a retrospective case series report. ESC heart failure. 2019 Apr;6(2):308-18.
4. Wintrich J, Pavlicek V, Brachmann J, Bosch R, Butter C, Oswald H, Rybak K, Mahfoud F, Böhm M, Ukena C. Remote Monitoring With Appropriate Reaction to Alerts Was Associated With Improved Outcomes in Chronic Heart Failure: Results From the OptiLink HF Study. Circ Arrhythm Electrophysiol. 2021 Jan;14(1):e008693. doi: 10.1161/CIRCEP.120.008693. Epub 2020 Dec 10. PMID: 33301362.
5. Volterrani M, Spoletini I, Angermann C, Rosano G, Coats AJ. Implantable devices for heart failure monitoring: the CardioMEMS™ system. Eur Heart J Suppl. 2019 Dec;21(Suppl M):M50-M53. doi: 10.1093/eurheartj/suz265. Epub 2019 Dec 31. PMID: 31908617; PMCID: PMC6937499.
6. Abraham WT, Perl L. Implantable Hemodynamic Monitoring for Heart Failure Patients. J Am Coll Cardiol. 2017;70(3):389-398. doi:10.1016/j.jacc.2017.05.052.
7. Schmier JK, Ong KL, Fonarow GC. Cost-Effectiveness of Remote Cardiac Monitoring With the CardioMEMS Heart Failure System. Clin Cardiol. 2017;40(7):430-436. doi:10.1002/clc.22696.
8. Shavelle D, Jermyn R. The CardioMEMS Heart Failure Sensor: A Procedural Guide for Implanting Physicians. J Invasive Cardiol. 2016;28(7):273-279.
9. Holy Cross Health. CardioMEMS Technology. Accessed October 20, 2023 from https://www.holy-cross.com/find-a-service-or-specialty/heart-and-vascular-care/treatments-and-procedures/cardiomems-technology.
10. CardioMEMS, Inc. CardioMEMS™ HF System. Published May 2014. Accessed October 20, 2023 from https://www.accessdata.fda.gov/cdrh_docs/pdf10/p100045d.pdf.
11. Chonde D. CardioMEMS device. Case study, Radiopaedia.org. Accessed October 20, 2023 from https://doi.org/10.53347/rID-70862.
12. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. The Lancet. 2011 Feb 19;377(9766):658-66.
13. Leung CC. Current Role of the CardioMEMS Device for Management of Patients with Heart Failure. Curr Cardiol Rep. 2019;21(9):98. Published 2019 Jul 27. doi:10.1007/s11886-019-1194-9.
14. Angermann CE, Assmus B, Anker SD, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF). Eur J Heart Fail. 2020;22(10):1891-1901. doi:10.1002/ejhf.1943.
15. Brugts JJ, Radhoe SP, Clephas PRD, et al. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF): a randomised clinical trial [published correction appears in Lancet. 2023 Jun 24;401(10394):2112]. Lancet. 2023;401(10394):2113-2123. doi:10.1016/S0140-6736(23)00923-6.
16. Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. 2021;398(10304):991-1001. doi:10.1016/S0140-6736(21)01754-2.
17. Guichard JL, Cowger JA, Chaparro SV, Kiernan MS, Mullens W, Mahr C, Mullin C, Forouzan O, Hiivala NJ, Sauerland A, Leadley K. Rationale and design of the proactive-HF trial for managing patients with NYHA class III heart failure by using the combined Cordella pulmonary artery sensor and the Cordella heart failure system. Journal of cardiac failure. 2023 Feb 1;29(2):171-80.








