Necrotizing Fasciitis: Pearls & Pitfalls
- Oct 22nd, 2015
- Jamie Santistevan
- categories:
Necrotizing Fasciitis
by Jamie Santistevan MD
Senior EM Resident Physician, University of Wisconsin
Edited by Alex Koyfman, MD (EM Attending Physician, UT Southwestern Medical Center / Parkland Memorial Hospital, @EMHighAK) and Stephen Alerhand, MD (@SAlerhand)
CASE
A 39-year-old woman presents to the ED with leg pain and fever. She initially noted redness and pain above her knee 2 weeks ago and was evaluated at an outside hospital. She completed a 10-day course of oral antibiotics for cellulitis. Over the last two days, she has had progressive leg swelling of her entire right thigh. The pain is now so severe that she is having difficulty walking. Her past medical history is negative for diabetes mellitus, chronic liver disease, or alcohol and IV drug use.
On exam, she is febrile to 102.7 F, heart rate is 96 bpm, and blood pressure is 112/65. She has a 12 cm area of faint erythema on her right thigh and tenderness to palpation of her entire right leg with diffuse edema. There is no ecchymosis or bullae formation.
She is admitted for IV antibiotics to treat cellulitis. Overnight, she complains of severe pain requiring multiple doses of narcotics. In the morning, a CT scan is obtained that demonstrates fluid and gas along the rectus femoris muscle. Surgery is consulted for debridement of necrotizing fasciitis.
Figure 1: CT scan demonstrating fluid and gas in the thigh
INTRODUCTION
Necrotizing soft tissue infections (NSTI) include necrotizing forms of cellulitis, fasciitis, and myositis. NSTI’s are rare but deadly deep soft tissue infections associated with tissue destruction, systemic toxicity, and high morbidity and mortality. The estimated mortality rate of necrotizing fasciitis is estimated to be between 25-35% [1, 2]. Necrotizing infections can occur anywhere on the body but most commonly affect the extremities, perineum, and genitalia, while rarely arising on the trunk [2, 3]. Infection requires inoculation with the bacteria, which typically occurs via a break in the epithelial or mucosal surface secondary to trauma, IV drug use, insect or animal bites, or surgery. However, it has also been reported without any known trauma in up to 17% of cases [4]. The rarity of the disease and lack of pathognomonic signs and symptoms early in its course make it challenging to diagnose. However, rapid diagnosis is essential because early surgical debridement reduces mortality and amputation rates [5, 6, 7, 8, 9].
Necrotizing skin infections are classified into three groups based on the type of bacteria present. Giuliano and colleagues first described this classification system in 1977 [10]. It is important to note that each group has different risk factors and “classic” patient demographics, though these classifications are not exclusive.
- Type I infections are polymicrobial, which include gram-positive cocci, gram-negative rods, and at least one anaerobic species [2]. Common anaerobes include Bacteroides, Clostridia species, Prevotella, or Peptostreptococcus [11, 12]. Gram-negative organisms include Pseudomonas (particularly in immunosuppressed patients or those with malignancy) and Enterobacteriaceae [12]. Type I infections remain the most common. Patients with Type I infections tend to be older with multiple medical comorbidities such as diabetes mellitus, chronic liver disease, obesity, immunosuppression, peripheral vascular disease, and recent surgery [4, 5, 7, 9, 11, 12]. Fournier’s Gangrene is an example of a Type I infection of the perineum with bacterial sources from the skin, colon, anus, and rectum including coli, Klebsiella, Enterococci, and anaerobes. Diabetes is a major risk factor for Fournier’s Gangrene, but it can also occur after vasectomy in adults or in neonates with omphalitis or following circumcision [12, 13]. Clostridial infections (aka gas gangrene) are included in this category, but have decreased in incidence over time due to improved sanitation, although they continue to be reported among IV drug users injecting black tar heroin subcutaneously [14]. Infection with Clostridia species is characterized by rapid onset of symptoms after inoculation and is an independent predictor for limb loss and mortality [3].
- Type II infections are monomicrobial, most commonly caused by group A beta-hemolytic Streptococcus alone or with aureus [2]. Patients tend to be younger and healthier and may have a history of trauma, laceration, burn, surgery, or IV drug use [8, 11, 12]. Necrotizing infections due to group A strep (GAS) can also be associated with toxic shock syndrome in about half of the cases [15, 16]. The virulence of GAS is determined by the M-protein, which has antiphagocytic properties [15, 16, 17]. Community-acquired methicillin-resistant S. aureus (MRSA) can also cause monomicrobial infection in communities with a high prevalence [18]. Risk factors included current or past IV drug use (in 43% of patients), previous MRSA infection, diabetes, chronic hepatitis C and cancer and HIV [18].
- Type III infections are caused by Gram-negative marine organisms. Infection generally occurs after an open wound or other break in skin is exposed to fresh or salt water. Aeromonas hydrophila is associated with wounds exposed to fresh water, whereas Vibrio vulnificus can occur in seawater injuries or in a patient who ingests or handles raw oysters [2, 19, 20]. Risk factors include alcoholism and liver cirrhosis [20]. Type III infections have been reported along warm water coastal regions in the US, Central and South America, and Asia [2, 20].
DIAGNOSIS
Necrotizing fasciitis (NF) is a necrotizing soft tissue infection of muscular fascia in which the muscle tissue below is frequently spared. Infection spreads quickly along the fascia due to poor blood supply. The skin findings seen on exam are secondary to vasculitis and thrombosis of the perforating blood vessels, which produces tissue ischemia, erythema, and cutaneous anesthesia. In order for skin findings to develop, a large number of capillary beds need to be involved. Therefore, the skin may only appear mildly infected superficially, whereas the infection beneath can be quite severe. Patients may not show signs of severe systemic illness until the disease progresses. In fact, NF is misdiagnosed as cellulitis in up to three quarters of the time [1, 8]. NF commonly occurs in obese, diabetic, or otherwise immunocompromised patients as described above, though it can also occur in young otherwise healthy patients without predisposing factors [2, 18]. To avoid misdiagnosis of this deadly disease, we will explore some pitfalls in clinical decision-making.
Diagnostic pitfalls
“Crepitus and bullae are absent, so it can’t be necrotizing fasciitis.”
Early signs and symptoms of NF are similar to those with simple cellulitis or abscess and can include pain, erythema, fever, and chills. More ominous signs include bullae, skin necrosis, pallor, hypoesthesia, and crepitus. These findings occur later in the course of the disease. Relying on the absence of skin necrosis, cutaneous anesthesia, gas formation, and bullae to rule out NF can be dangerous. Each of these “classic” physical exam findings is only present about 20-25% of the time [1]:
- Bullae
- Skin necrosis
- Crepitus
- Cutaneous anesthesia
- Septic shock
These findings are considered “hard” findings for necrotizing fasciitis and should prompt immediate surgical exploration. Subcutaneous emphysema has been traditionally stressed as the classic finding of NF, but only gas-forming organisms produce this finding, and it is only present between 13-31% of the time. As the infection progresses, the skin may demonstrate brown or bronze discoloration and bullae may form. Patients who present with these findings or in septic shock already have advanced disease [2].
“The LRINEC score was low, so it’s definitely not necrotizing fasciitis”.
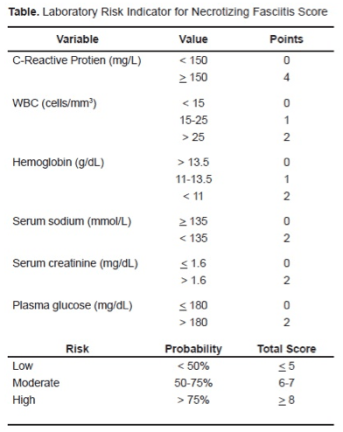
Laboratory studies in patients with necrotizing fasciitis may reveal metabolic acidosis, leukocytosis, anemia, thrombocytopenia, coagulopathy, hyponatremia, or renal or liver dysfunction. The LRINEC score (Laboratory Risk Indicator in Necrotizing Fasciitis) uses 6 laboratory parameters to estimate the patient’s risk of NF [TABLE 1].
The score was developed based on data from 89 patients with NSTI compared with 314 patients with severe cellulitis, abscess, or both. A score > or = 6 was found to have 92% positive predictive value and a 96% negative predictive value [22]. However, the score has limited sensitivity and therefore should not be used as the sole determinant of clinical decision-making for the diagnosis of NF. It has not been validated and other disease process may produce similar lab findings. There have been cases of confirmed NF with a LRINEC score of 0 [23], and it has been found to have only a small effect on post-test probability [24]. Therefore, interpret the score with caution because your clinical suspicion may be superior.
“An X-ray didn’t show free air, so I’m in the clear.”
In general, there is a limited role for imaging of suspected NF. The potential delay to definitive treatment could outweigh any benefit in helping to diagnose these infections. Not all organisms that cause NF produce gas, though when present, subcutaneous gas is highly specific for clostridial infections [2, 21]. The presence of gas on a plain film in confirmed NF is relatively low with a mean rate of 24.8% across studies [1]. Plain radiographs are not sensitive enough to safely exclude the diagnosis. If there is high clinical suspicion, surgical consultation is warranted.
“I can rely on an MRI or CT to secure the definitive diagnosis.”
CT and MRI are more sensitive than plain radiographs but lack specificity [2, 25, 26, 27]. This means that other disease processes may have similar findings. CT may show facial swelling, gas formation, or fluid collections. MRI is more sensitive but tends to overestimate myofascial involvement [26]. The use of IV contrast for CT scan may be limited by concomitant renal failure whereas MRI is costly, time-consuming, and not always readily available. Bedside or formal ultrasound may provide useful information by demonstrating hyperechoic foci with reverberation artifact at the site of infection (dirty shadowing), but this may be difficult to detect for the novice ultrasonographer. As stated before, if you are suspicious of NF, imaging should not delay surgical consultation.
Diagnostic Pearls
Despite the various pitfalls in making the diagnosis, there are some physical exam findings that can help make the diagnosis. The following are several clues to differentiate NF from cellulitis [1, 2, 21 25]:
- Pain out of proportion to skin findings
- Indistinct margins of involvement
- Tenderness and edema beyond the skin erythema
- Absence of lymphangitis
- Infection progresses despite antibiotics
Swelling and severe pain are the most common symptoms reported by patients with necrotizing fasciitis [1, 21]. Vital sign abnormalities may be present, fever and tachycardia being the most common [21]. In uncertain cases, the “finger test” can be performed whereby under local anesthesia an incision is made in the skin down to the deep fascia and the suspicious area is probed with the index finger. Positive results may include the presence of “dishwater pus”, the lack of bleeding, and the lack of tissue resistance to blunt dissection along the fascia [2, 25]. Of course, this is not a common technique most emergency physicians will employ. The gold standard for diagnosis is operative exploration. Surgical exploration is the only way to definitively establish the diagnosis and distinguish necrotizing fasciitis from other entities.
TREATMENT
The treatment of necrotizing fasciitis involves wide surgical debridement, broad-spectrum antibiotics, and hemodynamic support. The choice of antibiotics will vary based on the suspected organisms involved, as well as the local incidence of MRSA and drug susceptibilities. Most accepted regimens include coverage of gram-positive and gram-negative organisms and anaerobes. Here are some considerations for antibiotic selection based on the suspected type of infection and the organisms involved:
- Anaerobic coverage is important for suspected type I infection and can include metronidazole, clindamycin, or carbapenems [25].
- In patients who have recently been on antibiotics or been hospitalized, broad gram-negative coverage is necessary and can include ampicillin-sulbactam, piperacillin-tazobactam, ticarcillin-clavulanate, a third or fourth generation cephalosporin, or carbapenems.
- Type II infections will require treatment of pyogenes, typically with a beta-lactam antibiotic or clindamycin, but studies have shown that clindamycin is superior to penicillin for GAS infections [16, 28, 29, 30].
- Clindamycin has antitoxin effects against GAS and Clostridia species [14, 28, 29].
- Coverage of MRSA with vancomycin, daptomycin or linezolid should be based on local resistance patterns.
- If Vibrio vulnificus is suspected consider adding doxycycline, minocycline, or a third generation cephalosporin [25]
An acceptable regimen to initiate in the emergency department includes a carbapenem or beta-lactam-beta-lactamase inhibitor plus clindamycin and an agent directed against MRSA. Tissue ischemia will impair antibiotic delivery to the infected tissue and therefore, they are ineffective alone. Definitive treatment is source control with wide surgical debridement. Once the patient is hospitalized, antibiotics will be tailored based on gram stain, culture and sensitivity results. For GAS infections complicated by TSS, the administration of IVIG should be considered as an adjunctive therapy and has been shown to improve outcomes [31, 32, 33].
KEY POINTS
- Necrotizing fasciitis may be misdiagnosed as cellulitis due to the benign appearance of cutaneous findings early in the clinical course.
- Clues to the diagnosis of necrotizing fasciitis are vital sign abnormalities, swelling, and severe pain out of proportion to cutaneous findings.
- If you are suspicious for necrotizing fasciitis, have a low threshold to start broad-spectrum antibiotics and consult Surgery without delaying for advanced imaging, even if clinical score and plain films are unremarkable.
References / Further Reading
- Goh T, Goh LG, Ang CH, Wong CH. Early diagnosis of necrotizing fasciitis. Br J Surg. 2014 Jan;101(1):e119-25. PMID: 24338771
- Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: Review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014 Aug;51(8): 344–362. PMID: 25069713
- Anaya DA, Sullivan SR, Foy H, Bulger E. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg. 2005; 140:151–157. PMID: 15723996
- Childers B, Potyondy L, Nachreiner R. Necrotizing fasciitis: a fourteen-year retrospective study of 163 consecutive patients. Am Surg. 2002; 68(2):109–116. PMID: 11842952
- Freischlag J, Ajalat G, Busuttil R. Treatment of necrotizing soft-tissue infections. Am J Surg. 1985; 149:751–755. PMID: 4014552
- Voros D, Pissiotis C, Georgantas D, Al E. Role of early and extensive surgery in the treatment of severe necrotizing soft tissue infection. Br J Surg. 1993; 80:1190–1191. PMID: 8402129
- Bilton B, Zibari B, McMillan R, Al E. Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: a retrospective study. Am Surg. 1998; 64:397–400. PMID: 9585771
- Wong C, Haw-Chong C, Shanker P. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003; 85:1454–1460. PMID: 12925624
- Kalaivani V, Hiremath BV, Indumathi VA. Necrotising soft tissue infection-risk factors for mortality. J Clin Diagn Res. 2013 Aug;7(8):1662–5. PMID: 24086868
- Giuliano A, Lewis F, Hadley K, Blaisdell F. Bacteriology of necrotizing fasciitis. Am J Surg. 1977; 134(1):52–57. PMID: 327844
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705. PMID: 17278065
- Brook I, Frazier EH. Clinical and microbiological features of necrotizing fasciitis. J Clin Microbiol. 1995;33(9):2382. PMID: 7494032
- Hsieh WS, Yang PH, Chao HC, Lai JY. Neonatal necrotizing fasciitis: a report of three cases and review of the literature. Pediatrics. 1999;103(4):e53. PMID: 10103345
- Bryant AE, Stevens DL. Clostridial myonecrosis: new insights in pathogenesis and management. Curr Infect Dis Rep. 2010; 12(5):383–391. [PubMed: 21308521]
- Kaul R, McGeer A, Low DE, Green K, Schwartz B. Population-based surveillance for group A streptococcal necrotizing fasciitis: Clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am J Med. 1997;103(1):1. PMID: 9236481
- Stevens D. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1995; 1(3):69. PMID: 8903167
- Chelsom J, Halstensen A, Haga T, Høiby EA. Necrotising fasciitis due to group A streptococci in western Norway: incidence and clinical features. Lancet. 1994;344(8930):1111. PMID: 7934492
- Miller L, Carrick M, Scott B, Hodges J, Pham H. Necrotizing fasciitis caused by community associated methicillin-resistant Staphylococcal aureus in Los Angeles. N Engl J Med. 2005; 352(14):1445–1453. PMID: 15814880
- Hau V, Ho CO. Necrotising fasciitis caused by Vibrio vulnificus in the lower limb following exposure to seafood on the hand. Hong Kong Med J. 2011 Aug;17(4):335-7. PMID: 21813906
- Park K, Jung S, Jung Y, Shin J, Hwang J. Marine bacteria as a leading cause of necrotising fasciitis in coastal areas of South Korea. Am J Trop Med Hyg (2009) 80:646–50. PMID: 19346393
- Sudarsky LA, Laschinger JC, Coppa GF, Spencer FC. Improved results from a standardized approach in treating patients with necrotizing fasciitis. Ann Surg. 1987;206(5):661. PMID: 3314752
- Wong C, Khin L, Heng K, Tan K, Low C. The LRINEC (laboratory risk indicator for necrotising fasciitis) score: a tool for distinguishing necrotising fasciitis from other soft-tissue infections. Crit Care Med. 2004 Jul;32(7):1535-41. PMID: 15241098
- Wilson MP, Schneir AB. A case of necrotizing fasciitis with a LRINEC score of zero: clinical suspicion should trump scoring systems. J Emerg Med. 2013 May;44(5):928-31. PMID: 23287745
- Holland MJ. Application of the Laboratory Risk Indicator in Necrotising Fasciitis (LRINEC) score to patients in a tropical tertiary referral centre. Anaesth Intensive Care. 2009 Jul;37(4):588-92. PMID: 19681416
- Misiakos EP, Bagias G, Patapis P, Sotiropoulos D, Kanavidis P, Machairas A. Current concepts in the management of necrotizing fasciitis. Front Surg. 2014 Sep 29;1:36. PMID: 25593960
- Schmid MR, Kossmann T, Duewell S. Differentiation of necrotizing fasciitis and cellulitis using MR imaging. AJR Am J Roentgenol. 1998 Mar;170(3):615-20. PMID: 9490940
- Zacharias N, Velmahos GC, Salama A, Alam HB, de Moya M, King DR, Novelline RA. Diagnosis of necrotizing soft tissue infections by computed tomography. Arch Surg. 2010 May;145(5):452-5. PMID: 20479343
- Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096. PMID: 10608632
- Stevens D, Meier K, Mitten J. Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob Agents Chemother. 1987; 31(2):213–218. PMID: 2882731
- Stevens DL, Bryant AE, Yan S. Invasive group A streptococcal infection: new concepts in antibiotic treatment. Int J Antimicrob Agents 1994; 4:297-301. PMID: 18611620
- Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis. 2014;59(3):358. PMID 24785239
- Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Schwartz B, O’Rourke K, Talbot J, Low DE. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome–a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis. 1999;28(4):800. PMID 10825042
- Stegmayr B, Björck S, Holm S, Nisell J, Rydvall A, Settergren B. Septic shock induced by group A streptococcal infection: clinical and therapeutic aspects. Scand J Infect Dis. 1992;24(5):589. PMID: 1465576
- http://www.ncbi.nlm.nih.gov/pubmed/25786347
- http://www.ncbi.nlm.nih.gov/pubmed/25899752
- http://www.ncbi.nlm.nih.gov/pubmed/25671035


5 thoughts on “Necrotizing Fasciitis: Pearls & Pitfalls”