Pulmonary Hypertension: ED Presentation, Evaluation, and Management
- Jan 11th, 2021
- Rachel Bridwell
- categories:
Author: Rachel Bridwell, MD (@rebridwell, EM Resident Physician, San Antonio, TX) // Reviewed by: Brit Long, MD (@long_brit, EM Attending Physician, San Antonio, TX); Skyler Lentz, MD (@skylerlentz, Assistant Professor of Surgery (EM) and Medicine (Critical Care), University of Vermont Medical Center); Alex Koyfman, MD (@EMHighAK, EM Attending Physician, UTSW / Parkland Memorial Hospital)
Clinical Case #1
A 27 year old male presents to the ED with 6 months of cough and dyspnea. He reports recent bouts of hemoptysis. He has not traveled or been exposed to any high risk areas for tuberculosis. He states he had heart surgery as a baby but cannot recall what it was. He additionally reports previous bouts of epistaxis but none recently.
On exam his vitals are HR 105, BP 104/81, RR 24, 90% on room air, T 98.1
HEENT: No conjunctival pallor
Pulm: Increased work of breathing though no wheezes, rhonchi, rales
CV: Tachycardic, palpable S2, positive left parasternal heave
Integumentary: no telangiectasias
Clinical Case #2
A 57 year old male with a PMHx of pulmonary hypertension and congestive heart failure presents with shortness of breath and syncope. He recently ran out of his high dose sildenafil. He denies chest pain though states his symptoms worsen with walking. Denies recent cough, fever, abdominal pain. He is a daily tobacco user.
On exam his vitals are HR 115, BP 101/72, RR 28, 86% on room air, T 98.1
HEENT: No conjunctival pallor
Pulm: Increased work of breathing though no wheezes, rhonchi, rales
CV: Tachycardic, S3, no murmurs
Integumentary: No clubbing or pallor
Background
Pulmonary hypertension (PH), characterized by increased pulmonary vascular resistance and pulmonary arterial pressure, is associated with significant morbidity and mortality. The disease is heterogenous, with varying demographics and underlying etiologies, affecting 15-60 million individuals worldwide.1 PH is defined by a mean pulmonary arterial pressure of greater than 25 mm Hg by right heart catheterization or inferred by echocardiogram.2 Although rare, with an estimated 5-15 cases per 1 million adults, recent studies identify PH-associated complaints as responsible for 64,000 ED visits over a 5 year period.2 In the United States, the most common cause of PH is left sided heart failure.3,4 In 1973, the World Health Organization (WHO) defined a classification system for PH to design and implement international health standards. This was revised in 2013 to redefine groups 1-5 (Table 1).5,6

Pulmonary Pathophysiology
In the setting of PH, physiologic derangements represent changes in pulmonary and systemic circulation, altering the pressures in each system. The pulmonary circulation is normally a low-resistance, low-pressure system, composed of thin-walled vessels capable of accommodating vast alterations in preload. In patients with chronic PH, pulmonary vascular resistance gradually increases with vascular remodeling of the pulmonary arteries. This remodeling involves vascular smooth muscle and endothelial cell proliferation, inflammation, and fibrosis. If remodeling occurs over a prolonged period of time, the right ventricle (RV) is able compensate, however, in the setting of RV dilation, tricuspid regurgitation becomes common. Beyond a specific point of RV distension, RV output decreases secondary to increased pulmonary pressure and impedance to RV outflow. This derangement sets in motion a vicious cascade of interventricular dependence in which bulging of the RV into the left ventricle (LV) decreases LV filling, subsequently decreasing cardiac output (Fig. 1).7–10 As cardiac output falls, end organ perfusion suffers, further exacerbating the pathophysiology of the inherent underlying disease.9
Figure 1. Pathophysiology of PH.

Alterations in pulmonary system pressure and ventricular wall thickness additionally reduce coronary artery perfusion. In a normal physiologic setting, the coronary arteries of the RV are perfused in both systole and diastole due to low RV wall tension as compared to the LV.10 In PH, as RV pressures increase, RV perfusion falls until pulmonary artery pressures exceed systemic pressure, generating RV ischemia. In this under-perfused state, RV contractility declines, worsening RV overload.8,11,12 The emergency management of PH focuses on many of these pathways to combat these physiologic derangements to improve patient hemodynamic and respiratory status.
ED Evaluation
Patient Presentation
Accounting for approximately 5 to 15 cases per one million ED visits, PH and its associated symptoms are non-specific.4 Dyspnea is the most common presenting complaint, though patients also report fatigue, weakness, chest pain, and syncope.5,13 Symptomatology in undiagnosed PH is insidious; many individuals experience dyspnea and fatigue, worsening over weeks to months, eventually limiting daily activities. Angina results from ischemic subendocardial injury from ventilation-perfusion mismatch or compression of the left main coronary artery by the pulmonary trunk.13 Syncope is also associated with a poor prognosis, while hoarseness may occur due to compression of the recurrent laryngeal nerve by an enlarged pulmonary artery.5,13 In advanced disease, individuals frequently present with symptoms of right heart failure (RHF): ascites, peripheral edema, and hemoptysis.13,14 Emergency clinicians should evaluate for underlying precipitants of RHF including sepsis, unplanned withdrawal of PH therapy, medication non-compliance, pregnancy, pneumonia, anemia, and arrhythmias.41,15
History
In individuals absent a previous diagnosis of PH, initial history should include PH risk factors including: congenital heart disease, left-sided heart disease, valvular disease, pulmonary disease, connective tissue disease, liver disease, blood dyscrasias, thyroid disorders, dialysis-dependent renal disease, malignancy, stimulant use, and family history of PH.13,16 If available, a prior echocardiogram should be reviewed for signs of PH. In patients diagnosed with PH, questions regarding current therapy and medication compliance are essential to guide treatment and specialty consultation; therapeutic side effects of PH medications should be considered diagnoses of exclusion.
Physical Examination Findings
Physical examination is unreliable for determining the presence of PH in early disease stages. In advanced PH, signs of RHF are commonly present: elevated jugular venous pressure, hepatojugular reflex, ascites, hepatomegaly, and peripheral edema.5,13,16 On auscultation, an increased pulmonic component of the second heart sound (P2), an RV gallop, or the murmur of tricuspid regurgitation may be present. In PH patients, arrhythmias including atrial fibrillation, atrial flutter, and atrioventricular node re-entry tachycardias are common.40 In one systematic review, authors found that although a number of the aforementioned signs had high specificities (88% for an S4 on inspiration, 85% for a loud P2 on inspiration, 84% for an RV lift on inspiration), their sensitivities were low (12%, 29%, and 21%, respectively).17 The physical examination finding with the highest positive likelihood ratio was a loud P2 on inspiration (LR+ 1.9, 95% Credible Interval 1.2-3.1).17 Patients with decompensated PH frequently present with hypotension, displaying signs of systemic hypoperfusion including diaphoresis, cool extremities, peripheral cyanosis, and tachycardia. Patients with a patent foramen ovale who suffer from PH may exhibit symptoms of right-to-left shunting, manifesting as systemic hypoxemia and cyanosis not corrected by supplemental oxygen.18,19
Studies
An electrocardiogram (ECG) should be obtained which in PH will reveal right axis deviation and right ventricular hypertrophy (Figure 2).15,20 In addition to a tachyarrhythmia, right atrial enlargement, and ST segment depression and T wave inversion in the precordial leads may show right heart strain.16
Figure 2. ECG with evidence of pulmonary hypertension.

Given the numerous etiologies of PH, laboratory studies should be guided by the patient presentation, history, and examination findings. In patients with chronic PH, venous blood gas frequently demonstrates hypoxemia and respiratory alkalosis.13,16 Brain Natriuretic Protein (BNP) measurement may be useful in narrowing the differential diagnosis of a patient presenting with dyspnea.20 BNP levels > 400 pg/ml suggest heart failure, but do not exclude other underlying conditions.21 Normal plasma BNP levels increase with age and are higher in women than in men.22 In the setting of renal failure, BNP should be interpreted with caution as reduced clearance may lead to chronic elevation.20 Evaluation of myocardial perfusion and end-organ function is critical in patients presenting with signs and symptoms consistent with heart failure, prompting laboratory evaluation of troponin, renal function panel, liver function panel, and lactate, as an elevated troponin and liver function tests portend a poor prognosis.21 The degree of heart failure is reflected by elevations in AST, ALT, and total bilirubin signify a decreased cardiac index and increased central venous pressure.22,23
Imaging narrows the differential diagnosis among etiologies of PH and guides resuscitation, which should include a chest radiograph and bedside echocardiography (Figure 3). Radiographic findings of PH include dilated pulmonary arteries, peripheral pruning, and an enlarged right atria and RV, while pleural effusions may reflect severe disease.16 While chest radiography demonstrates high sensitivity (96.9%) and specificity (99.1%) for detecting severe PH, it lacks sufficient sensitivity for detecting mild PH.24
In the setting of PH, bedside echocardiography will review right-sided pressure overload: right atrial enlargement, RV dilation (RV: LV > 1:1; normal < 0.6), increased RV free wall thickness (> 5-7 mm as measured at end-diastole by M-mode or 2D echocardiography from the parasternal long axis or subxiphoid view), end-systolic flattening of the intraventricular septum, and interventricular interdependence visualized as a “D”-shaped left ventricle in diastole (Figure 3).25 Ultrasound assessment of the inferior vena cava (IVC) may be misleading in PH patients, especially those with mitral regurgitation or aortic stenosis (Group 2 PH), revealing plethora that may not reflect intravascular volume status.10,26
Figure 3. Ultrasound depicting RV dilatation.

Computed Tomography (CT) plays an essential role in identifying potential etiologies underlying PH (Figure 4). A CT demonstrating right atrial enlargement, RV dilation main pulmonary/ascending aorta diameter ratio ³ 1 is suggestive of PH, with a positive predictive value of 96% (Figure 4).27 On CT, the pulmonary trunk should be no larger than 2.8 cm at the level of its bifurcation. Measurements > 2.8 cm suggest PH with a sensitivity of 69%–87% and a specificity of 89%–100%.28 For individuals with CTEPH, CT angiography may reveal thrombi in the pulmonary vasculature and identify shunts contributing to the patient’s presentation. In this patient population, non-enhanced CT may reveal a mosaic pattern of variable attenuation in the lung parenchyma with evidence of irregular pulmonary perfusion due to chronic thromboemboli.
Figure 4. CT of the chest demonstrating evidence of PH with a main pulmonary/ascending aorta diameter ratio ³ 1 and a pulmonary trunk of >2.8 cm.
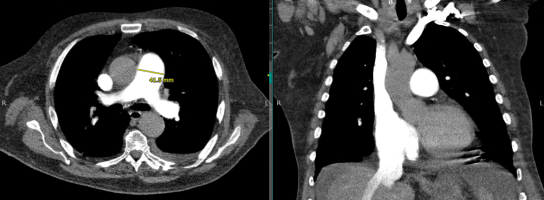

LV dysfunction is suggested on CT by the presence of a mosaic pulmonary perfusion pattern and pulmonary ground-glass opacities, demonstrating chronic pulmonary edema. The use of high-resolution CT in patients with PAH, without co-existing lung disease, should demonstrate normal lung parenchyma. Interstitial pulmonary abnormalities revealed by CT may point to an intrinsic lung disease as the etiology of the PH.
ED Management
The primary goal of the emergency clinician is the identification and treatment of the underlying etiologies of PH (e.g. alveolar hypoxia in COPD, hyperthyroidism, etc.). For individuals presenting with RHF, triggering factors should be addressed: antibiotic therapy administered for infections, transfusions given as indicated for anemia to maintain a hemoglobin of greater than 10 g/dL, and arrhythmias treated.22,29 New-onset atrial fibrillation or flutter are common in PH patients. In the absence of randomized controlled trials, observational studies suggest improved mortality with a rhythm control strategy.30 Rate control with calcium channel blockers or b-blockers is not advised, as these medications further impair RV function.22 In particular, b-blockers should be avoided as they may result in systemic hypotension through negative inotropic and chronotropic effects.31 Although digoxin may slow the ventricular rate in patients with supraventricular tachyarrhythmias associated with RV dysfunction, cardioversion is the preferred therapy for PH patients given this population’s propensity for digoxin toxicity.32 Electrical cardioversion is favored as prolonged atrial arrhythmias in patients with PH are associated with rapid decompensation. Pharmacologic cardioversion with Class III agents, such as amiodarone and sotalol, have been reported.33–35 While Class IC agents may theoretically be utilized, data in PH patients is lacking. In individuals with atrial arrhythmias lasting > 48 hours, anticoagulation is advised prior to cardioversion.30
In PH patients who present due to an unexpected discontinuation in oral or IV PH therapy, every effort should be made to contact the patient’s PH specialist to initiate ED treatment and prevent acute decompensation. While prostacyclins, endothelin receptor agonists, and phosphodiesterase inhibitors may not be immediately available in the ED, initiating these therapies early in the ED course may help to stave off clinical instability, though optimizing oxygenation and circulation should take priority.36–38 In individuals with PH, supplemental oxygen is indicated to maintain an oxygen saturation > 90%.22,39 Hypercapnia should be avoided as it results in further pulmonary vasoconstriction.6,41,22,39 Continuous non-invasive positive pressure ventilation may be considered, though fluid balance must be optimized prior to initiation to eliminate dangerous decreases in cardiac output.22 Although data are limited in the setting of acute exacerbations, non-invasive positive pressure ventilation in Group 3 PH patients with no LV dysfunction is typically well tolerated and improves hypercapnia.40,41 There are no current recommendations regarding the use of bilevel positive pressure ventilation in PH patients given the paucity of data.610,41 High-flow nasal cannula (HFNC) is an alternative therapy that may improve hypoxemia, especially if patients are unable to tolerate the mask utilized for NIPPV.
Intubation should be avoided if possible, as the effect of sedatives and positive intrathoracic pressure may reduce preload, cardiac function and cause peripheral vasodilation, resulting in hypotension and cardiovascular collapse.10 If intubation is required, etomidate is recommended for induction, given its limited effects on cardiac contractility and vascular tone. Hemodynamic optimization prior to intubation is recommended. If the patient is hemodynamically unstable, vasopressors should be initiated before attempts to establish a definitive airway are made.21,42 Awake intubation with topical anesthetics is an alternative that should be considered given the reduced risk of hemodynamic decompensation as compared to rapid sequence intubation. Medications administered during rapid sequence induction will likely result in profound hemodynamic collapse, hypercarbia, hypoxemia, and acidosis.10 Ventilator settings should target 6-8 mL/kg of ideal body weight and plateau pressures less than 30 cm H2O. Low positive end expiratory pressures should be utilized to minimize decreases in preload and increases in RV afterload.8,21 Hypoxemia and hypercapnia should be avoided.10,16,51,58
In the majority of cases, RV failure will be associated with fluid overload.32 IV diuretics should be used cautiously to obtain a negative fluid balance, optimizing circulating blood volume and reducing RV preload, and thus improving cardiac output.43 For patients not previously receiving oral diuretics, an initial dose of 20 – 40 mg IV furosemide is recommended for hypervolemia.43 In individuals utilizing home diuretic therapy, the initial IV dose should be at least equivalent to the oral dose.42 Consultation may be required for ultrafiltration in patients who are resistant to diuretic therapy.42 In hypovolemic patients, volume should be delivered conservatively, with boluses of 250 mL over 15-30 minutes.43
In the setting of hemodynamic instability, vasopressors should be initiated. Vasopressors increase aortic root pressure, increasing RV perfusion.10 Norepinephrine is an effective first-line vasopressor for patients with PH.43–45 Although norepinephrine primarily targets a1 receptors, with limited b1 stimulation to increase cardiac contractility, studies in heart failure patients have demonstrated improved RV myocardial oxygen delivery following administration (dose: 0.01-0.03mg/kg/min IV).44 The addition of low dose vasopressin (0.01-0.03 U/min) may be considered if the aforementioned therapies fail to result in hemodynamic improvement. Low doses of vasopressin may cause a decrease in pulmonary vascular resistance while beneficially raising the systemic blood pressure, proving uniquely beneficial in this action.46 Higher doses should be avoided (>0.08 U/min), which result in in pulmonary and coronary vasoconstriction.44,75
Epinephrine may benefit PH patients given the combined alpha and beta stimulation which provides system vascular support with inotropy, though this may increase myocardial oxygen demand. To date, there have been no trials regarding epinephrine use in adult PH patients. In a pilot study of hemodynamically stable children, epinephrine demonstrated an increase in systemic vascular resistance, though it worsened the systolic pulmonary artery pressure: aortic pressure ratio.47 Additionally, two patients experienced brief dysrhythmias, atrial bigeminy and ventricular bigeminy following administration of epinephrine.47 Animal models suggests that epinephrine improves cardiac index through inotropy, with systemic vascular resistance increasing in a dose dependent fashion.47,48 In contrast, sole a stimulation from phenylephrine should not be used in the unstable PH patient as it increases pulmonary vascular resistance, and may induce reflex bradycardia.49 Dopamine should be also avoided given b2-mediated decreases in systemic vascular resistance and possible arrhythmias.44
Inotropes increase the risk of tachyarrhythmias and should only be utilized in the setting of inadequate oxygen delivery, despite correction of abnormalities in RV preload and conditions causing RV ischemia.44 If inotrope support is required, dobutamine is the agent of choice.42,43 At low doses (5-10 mg/kg/min), dobutamine improves RV contractility and increases cardiac output through b1 receptor stimulation.42,43 Higher doses should be avoided, given increased stimulation of b2 receptors, causing vasodilation and hypotension.43 Milrinone, a selective PDE-3 inhibitor, is recommended for PH resulting from biventricular failure (0.375-0.75 mg/kg/min IV). Use in the ED may be limited given the requirement for pharmacy preparation and the drug’s slow onset of action.43 Milrinone improves inotropy and pulmonary vasodilation, but similar to dobutamine, it may cause hypotension.42,43
There are several other therapeutic options for patients with PH. Although sildenafil reduces RV afterload through pulmonary and systemic vasodilation, its use in critically ill patients in the ED is limited given the risk of hypotension. Patients who fail to respond to inotrope and vasopressor therapy should be considered for venoarterial extracorporeal membrane oxygenation.43,49 Patients with PH have a high risk of sudden cardiac death and poor outcomes. Cardiopulmonary resuscitation outcomes in PH patients with RV failure are poor: in a cohort of 3,130 PH patients who required CPR, only 6% survived for more than 90 days.21,50–52
Special Population – Prostacyclin Agonist Pump
A life-threatening emergency can occur if the patient has been prescribed IV epoprostenol or treprostinil and the IV catheter is removed or damaged, or the pump stops working.53,54 A PH specialist should be consulted emergently, and if possible, the pump should not be turned off as this may result in sudden death. If there is a problem with the line, pump, or cassette, peripheral access should be obtained and the pump tubing connected directly to this access.37,53,54 The line should not be primed or flushed, as a bolus of prostacyclin agonist may be delivered to the patient, resulting in fatal hypotension. The patient’s catheter should be inspected for drainage or surrounding cellulitis, which suggest infection. If administering medications or drawing laboratory studies, a second peripheral IV is required. An alternative infusion pump should not be utilized unless advised by the PH specialist.53,54
Inhaled Therapies
With low systemic absorption, inhaled vasodilators, e.g. nitric oxide and epoprostenol, decrease pulmonary vascular resistance, bettering cardiac output.55 These can be administered via endotracheal tube, NIPPV or HFNC, though depend on local hospital policy whether a closed circuit is required.56 However, these should be avoided in patients with LV failure and only initiated in conjunction with PH experts.55
Pulmonary Hypertension Crisis
Patients with PH may present unstable from an acute illness (e.g. sepsis) or become unstable after an intervention (e.g. intubation). The emergency clinician must have an approach to managing the patient with PH who becomes unstable to prevent the deadly cycle of hypotension, worsening pulmonary vascular resistance, RV ischemia worsening cardiac output leading to cardiovascular collapse. Below is a reference table to outline critical actions to take in a pulmonary hypertension crisis.

Disposition
If new onset PH, worsening or decompensated PH, or pump malfunction with prostacyclin agonist contribute to the ED presentation, admission and consultation with the specialty team are warranted. A formal transthoracic echocardiography is recommended to characterize RV size and function, and measure pulmonary artery systolic pressure and tricuspid jet.14,16
Key Points:
-Myriad of chief complaints can be pulmonary hypertension; try to determine what is triggering the acute decompensation.
–Elevations in troponin and liver function tests portend a poor prognosis.
–Point of care ultrasound may be useful in guiding acute resuscitation, though evaluation of IVC may not reflect intravascular volume.
–Avoid hypoxemia and hypercarbia and maintain right ventricular preload support.
-Most patients will be admitted.
–Avoid intubating these patients if at all possible.
–Restart PAH meds if discontinued.
References / Further Reading
- Delcroix M, Howard L. Pulmonary arterial hypertension: The burden of disease and impact on quality of life. Eur Respir Rev. 2015;24(138):621-629. doi:10.1183/16000617.0063-2015
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37(1):67-119. doi:10.1093/eurheartj/ehv317
- Stein PD, Matta F, Hughes PG. Scope of Problem of Pulmonary Arterial Hypertension. Am J Med. 2015;128(8):844-851. doi:10.1016/j.amjmed.2015.03.007
- Rich JD, Rich S. Clinical diagnosis of pulmonary hypertension. Circulation. 2014;130(20):1820-1830. doi:10.1161/CIRCULATIONAHA.114.006971
- Rose-Jones L, Mclaughlin V. Pulmonary Hypertension: Types and Treatments. Curr Cardiol Rev. 2014;11(1):73-79. doi:10.2174/1573403×09666131117164122
- Wilkins MR, Wharton J, Grimminger F, Ghofrani HA. Phosphodiesterase inhibitors for the treatment of pulmonary hypertension. Eur Respir J. 2008;32(1):198-209. doi:10.1183/09031936.00124007
- Santamore WP, Dell’Italia LJ. Ventricular interdependence: Significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis. 1998;40(4):289-308. doi:10.1016/S0033-0620(98)80049-2
- Green EM, Givertz MM. Management of acute right ventricular failure in the intensive care unit. Curr Heart Fail Rep. 2012;9(3):228-235. doi:10.1007/s11897-012-0104-x
- Maeder MT, Holst DP, Kaye DM. Tricuspid Regurgitation Contributes to Renal Dysfunction in Patients With Heart Failure. J Card Fail. 2008;14(10):824-830. doi:10.1016/j.cardfail.2008.07.236
- Wilcox SR, Kabrhel C, Channick RN. Pulmonary hypertension and right ventricular failure in emergency medicine. Ann Emerg Med. 2015;66(6):619-628. doi:10.1016/j.annemergmed.2015.07.525
- Watts JA, Marchick MR, Kline JA. Right Ventricular Heart Failure From Pulmonary Embolism: Key Distinctions From Chronic Pulmonary Hypertension. J Card Fail. 2010;16(3):250-259. doi:10.1016/j.cardfail.2009.11.008
- Zamanian RT, Haddad F, Doyle RL, Weinacker AB. Management strategies for patients with pulmonary hypertension in the intensive care unit. Crit Care Med. 2007;35(9):2037-2050. doi:10.1097/01.CCM.0000280433.74246.9E
- Sztrymf B, Souza R, Bertoletti L, et al. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J. 2010;35(6):1286-1293. doi:10.1183/09031936.00070209
- Medarov BI, Judson MA. The role of calcium channel blockers for the treatment of pulmonary arterial hypertension: How much do we actually know and how could they be positioned today? Respir Med. 2015;109(5):557-564. doi:10.1016/j.rmed.2015.01.004
- Maisel A. B-type natriuretic peptide levels: Diagnostic and prognostic in congestive heart failure: What’s next? Circulation. 2002;105(20):2328-2331. doi:10.1161/01.CIR.0000019121.91548.C2
- Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-1898. http://www.ncbi.nlm.nih.gov/pubmed/17168346. Accessed November 21, 2019.
- Colman R, Whittingham H, Tomlinson G, Granton J. Utility of the Physical Examination in Detecting Pulmonary Hypertension. A Mixed Methods Study. Bogaard H, ed. PLoS One. 2014;9(10):e108499. doi:10.1371/journal.pone.0108499
- Homma S, Di Tullio MR. Patent foramen ovale and stroke. J Cardiol. 2010;56(2):134-141. doi:10.1016/j.jjcc.2010.05.008
- Vaidya K, Khandkar C, Celermajer D. Current management aspects in adult congenital heart disease: Non-surgical closure of patent foramen ovale. Cardiovasc Diagn Ther. 2018;8(6):739-753. doi:10.21037/cdt.2018.09.09
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide concentration: Impact of age and gender. J Am Coll Cardiol. 2002;40(5):976-982. doi:10.1016/S0735-1097(02)02059-4
- Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med. 2011;184(10):1114-1124. doi:10.1164/rccm.201104-0662CI
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. In: Journal of the American College of Cardiology. Vol 43. Elsevier USA; 2004:S40-S47. doi:10.1016/j.jacc.2004.02.032
- van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA. Abnormal Liver Function in Relation to Hemodynamic Profile in Heart Failure Patients. J Card Fail. 2010;16(1):84-90. doi:10.1016/j.cardfail.2009.08.002
- Miniati M, Monti S, Airò E, et al. Accuracy of chest radiography in predicting pulmonary hypertension: A case-control study. Thromb Res. 2014;133(3):345-351. doi:10.1016/j.thromres.2013.12.019
- Markley RR, Ali A, Potfay J, Paulsen W, Jovin IS. Echocardiographic Evaluation of the Right Heart. J Cardiovasc Ultrasound. 2016;24(3):183. doi:10.4250/jcu.2016.24.3.183
- Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834-1837. doi:10.1007/s00134-004-2233-5
- Shen Y, Wan C, Tian P, et al. CT-base pulmonary artery measurementin the detection of pulmonary hypertension. Med (United States). 2014;93(27). doi:10.1097/MD.0000000000000256
- Kuriyama K, Gamsu G, Stern RG, Cann CE, Herfkens RJ, Brundage BH. CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol. 1984;19(1):16-22. doi:10.1097/00004424-198401000-00005
- Ruiter G, Lankhorst S, Boonstra A, et al. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J. 2011;37(6):1386-1391. doi:10.1183/09031936.00100510
- Wanamaker B, Cascino T, McLaughlin V, Oral H, Latchamsetty R, Siontis KC. Atrial arrhythmias in pulmonary hypertension: Pathogenesis, prognosis and management. Arrhythmia Electrophysiol Rev. 2018;7(1):43-48. doi:10.15420/aer.2018.3.2
- Perros F, de Man FS, Bogaard HJ, et al. Use of β-Blockers in Pulmonary Hypertension. Circ Hear Fail. 2017;10(4):e003703. doi:10.1161/CIRCHEARTFAILURE.116.003703
- Patel R, Aronow WS, Patel L, et al. Treatment of pulmonary hypertension. Med Sci Monit. 2012;18(4):RA31-RA39. doi:10.12659/MSM.882607
- Cannillo M, Grosso Marra W, Gili S, et al. Supraventricular Arrhythmias in Patients with Pulmonary Arterial Hypertension. Am J Cardiol. 2015;116(12):1883-1889. doi:10.1016/j.amjcard.2015.09.039
- Olsson KM, Nickel NP, Tongers J, Hoeper MM. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int J Cardiol. 2013;167(5):2300-2305. doi:10.1016/j.ijcard.2012.06.024
- Wen L, Sun ML, An P, et al. Frequency of supraventricular arrhythmias in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol. 2014;114(9):1420-1425. doi:10.1016/j.amjcard.2014.07.079
- Duarte JD, Hanson RL, Machado RF. Pharmacologic treatments for pulmonary hypertension: Exploring pharmacogenomics. Future Cardiol. 2013;9(3):335-349. doi:10.2217/fca.13.6
- Hohsfield R, Archer-Chicko C, Housten T, Harris Nolley S. Pulmonary arterial hypertension emergency complications and evaluation: Practical guide for the advanced practice registered nurses in the emergency department. Adv Emerg Nurs J. 2018;40(4):246-259. doi:10.1097/TME.0000000000000210
- Palevsky HI, Fishman AP. Chronic cor pulmonale. Etiology and management. JAMA. 1990;263(17):2347-2353. http://www.ncbi.nlm.nih.gov/pubmed/2182919. Accessed November 20, 2019.
- Galiè N, Rubin L, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371(9630):2093-2100. doi:10.1016/S0140-6736(08)60919-8
- Parola D, Romani S, Petroianni A, Locorriere L, Terzano C. Treatment of acute exacerbations with non-invasive ventilation in chronic hypercapnic COPD patients with pulmonary hypertension. Eur Rev Med Pharmacol Sci. 2012;16(2):183-191. http://www.ncbi.nlm.nih.gov/pubmed/22428469. Accessed November 23, 2019.
- Bento AM, Cardoso LF, Tarasoutchi F, Sampaio RO, Kajita LJ, Lemos Neto PA. Hemodynamic effects of noninvasive ventilation in patients with venocapillary pulmonary hypertension. Arq Bras Cardiol. 2014;103(5):410-417. doi:10.5935/abc.20140147
- Pritts CD, Pearl RG. Anesthesia for patients with pulmonary hypertension. Curr Opin Anaesthesiol. 2010;23(3):411-416. doi:10.1097/ACO.0b013e32833953fb
- Savale L, Weatherald J, Jaïs X, et al. Acute decompensated pulmonary hypertension. Eur Respir Rev. 2017;26(146). doi:10.1183/16000617.0092-2017
- Harjola VP, Mebazaa A, Čelutkiene J, et al. Contemporary management of acute right ventricular failure: A statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail. 2016;18(3):226-241. doi:10.1002/ejhf.478
- Bertolissi M, Bassi F, Da Broi U. Norepinephrine can be useful for the treatment of right ventricular failure combined with acute pulmonary hypertension and systemic hypotension. A case report. Minerva Anestesiol. 2001;67(1-2):79-84.
- Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: Current and emerging options for management: A systematic literature review. Crit Care. 2010;14(5):1-22. doi:10.1186/cc9264
- Siehr SL, Feinstein JA, Yang W, Peng LF, Ogawa MT, Ramamoorthy C. Hemodynamic effects of phenylephrine, vasopressin, and epinephrine in children with pulmonary hypertension: A pilot study. Pediatr Crit Care Med. 2016;17(5):428-437. doi:10.1097/PCC.0000000000000716
- Hyldebrandt JA, Sivén E, Agger P, et al. Effects of milrinone and epinephrine or dopamine on biventricular function and hemodynamics in an animal model with right ventricular failure after pulmonary artery banding. Am J Physiol Circ Physiol. 2015;309(1):H206-H212. doi:10.1152/ajpheart.00921.2014
- Ventetuolo CE, Klinger JR. Management of acute right ventricular failure in the intensive care unit. Ann Am Thorac Soc. 2014;11(5):811-822. doi:10.1513/AnnalsATS.201312-446FR
- Demerouti EA, Manginas AN, Athanassopoulos GD, Karatasakis GT. Complications leading to sudden cardiac death in pulmonary arterial hypertension. Respir Care. 2013;58(7):1246-1254. doi:10.4187/respcare.02252
- Delcroix M, Naeije R. Optimising the management of pulmonary arterial hypertension patients: Emergency treatments. Eur Respir Rev. 2010;19(117):204-211. doi:10.1183/09059180.00004910
- Hoeper MM, Galié N, Murali S, et al. Outcome after cardiopulmonary resuscitation in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165(3):341-344. doi:10.1164/ajrccm.165.3.200109-0130c
- Ruan CH, Dixon RAF, Willerson JT, Ruan KH. Prostacyclin therapy for pulmonary arterial hypertension. Texas Hear Inst J. 2010;37(4):391-399. doi:10.1007/978-1-60327-075-5_12
- (No Title). https://phassociation.org/wp-content/uploads/2017/02/school-resource-guide-Emergency-101-for-EMTs.pdf. Accessed November 21, 2019.
- Hill NS, Preston IR, Roberts KE. Inhaled therapies for pulmonary hypertension. Respir Care. 2015;60(6):794-802. doi:10.4187/respcare.03927
- Tremblay JA, Couture ÉJ, Albert M, et al. Noninvasive Administration of Inhaled Nitric Oxide and its Hemodynamic Effects in Patients With Acute Right Ventricular Dysfunction. J Cardiothorac Vasc Anesth. 2019;33(3):642-647. doi:10.1053/j.jvca.2018.08.004
