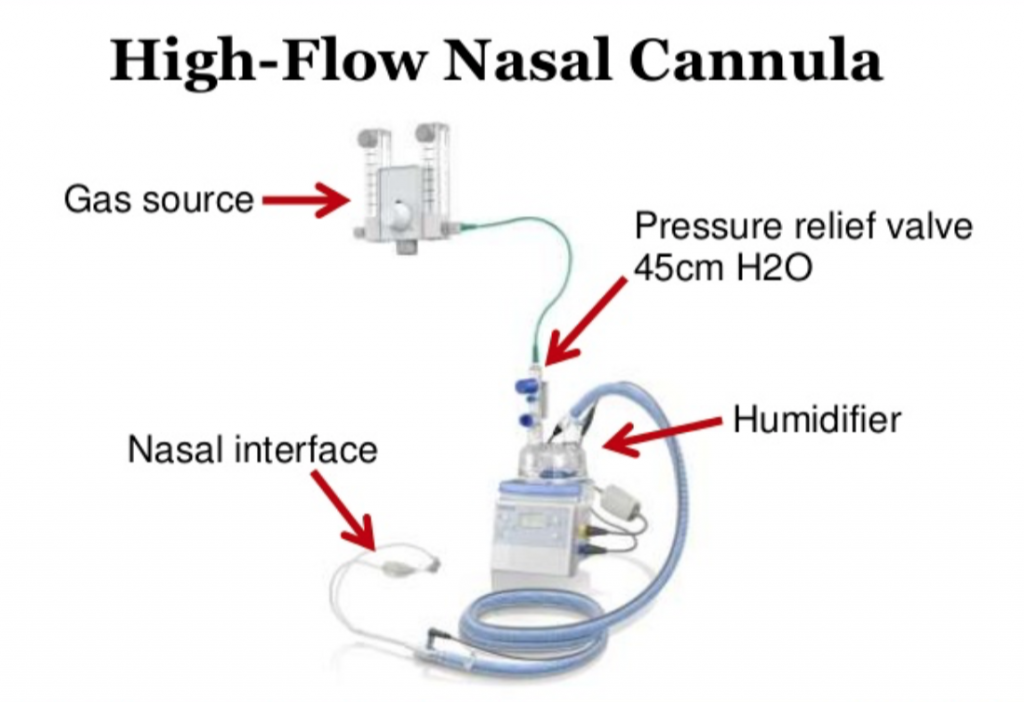
52 in 52 – #32: Hydrocortisone in Severe Community-Acquired Pneumonia
This week the ’52 in 52′ series covers hydrocortisone in patients with severe community-acquired pneumonia, or the CAPE-COD trial.
52 in 52 – #32: Hydrocortisone in Severe Community-Acquired Pneumonia Read More »