Authors: Tony Spadaro, MD, MPH (EM Resident Physician at the Hospital of the University of Pennsylvania) and Kevin R. Scott, MD, MSEd (EM Attending Physician at the Hospital of the University of Pennsylvania) // Reviewed by: Marina Boushra, MD; Alex Koyfman, MD (@EMHighAK); Brit Long, MD (@long_brit)
Case 1
A 37-year-old female presents with unilateral right-sided headache, nausea, and vomiting that developed gradually over the past week. She complains of associated photophobia and has had no improvement with over-the-counter medications. She denies any prior history of migraines. Her vital signs are BP 136/78, HR 62, RR 14, SpO2 100%, Temp 98.6 oF. Her physical examination is notable for anisocoria without any neurological abnormalities. In the emergency department (ED), her symptoms improve with ketorolac, and metoclopramide and she is eager to be discharged to return to her newborn.
Headache is one of the top 10 most common presenting complaints in the emergency department and often requires minimal laboratory or radiographic diagnostic work up. However, there are some red flags that should raise suspicion for serious pathology and prompt further investigation.1 Although a severe, sudden “thunderclap” headache will alert most clinicians to evaluate for a subarachnoid hemorrhage, clinicians should also consider other rare but dangerous causes of headache and the associated signs and symptoms that require further evaluation.1,2 Cerebral venous thrombosis (CVT) is one of these rare causes of headache that carries significant morbidity and mortality.2
Overview of CVT
CVT is defined by thrombosis of the intracranial veins and dural sinuses and has an estimated annual incidence of 0.3-1.5 cases/100,000 person-years, accounting for up to 1% of all strokes worldwide.3,4,5 It often occurs in patients who are young and lack traditional risk factors for ischemic stroke.3 A systematic review of 8829 patients found the mean age of patients with CVT to be 32.9 years.6 In fact, only 10% of cases occur in patients over 60 years of age.4,5,6 There is a female predominance, with approximately 2/3 of cases occurring in women.5,6
In order to understand the pathophysiology of the disease it is important to review the role of the cerebral veins and dural sinuses in the central nervous system. The cerebral veins drain the capillary network that supplies the brain with blood.7 The dural sinuses also drain the cerebrospinal fluid (CSF) via the arachnoid granulations and return the CSF into circulation through the blood stream.7 An obstruction within this drainage system leads to increased venous pressure and reduced capillary perfusion pressure, which can eventually result in vasogenic edema, elevated intracranial pressure (ICP), and even hemorrhagic infarction.3,7 Compression of the nerve fibers within the veins is thought to cause headache, which is a relatively common presenting complaint in CVT.5,6,7 Other possible presenting complaints can include focal neurologic deficits, altered mental status, or seizure.3
CVT most often occurs in patients who are in a hypercoagulable state. Specifically, CVT can be seen in individuals with factor V Leiden mutation, patients on estrogen-containing oral contraceptive pills (OCP), or those that are in the puerperium. Other risk factors include inflammatory conditions such as vasculitis or connective tissue disorders.5 While 85% of cases have at least one risk factor, up to 15% of cases have no identifiable risk factor.8,9
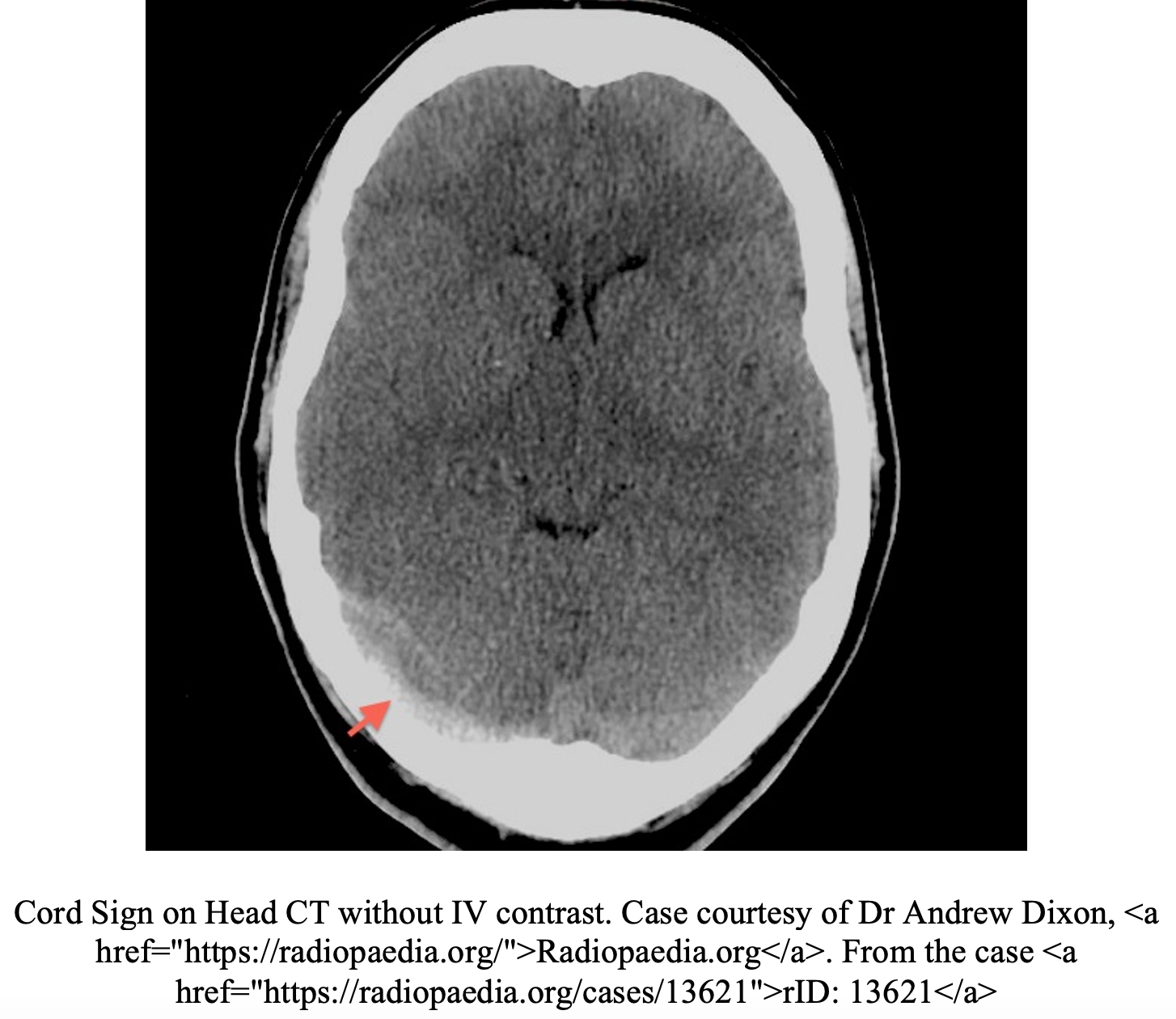
CVT is primarily diagnosed by imaging.1,2,9 Often, the first imaging test obtained in the emergency department in patients with suspected high-risk headaches is non-contrast head CT. Unfortunately, this test is poorly sensitive for CVT and can be normal in up to 30% of patients.10,11 Additionally the classic radiographic signs of CVT are only present in 25% of cases.5,10,11 Non-contrast head CT findings concerning for CVT include the “dense triangle” (clot in the superior sagittal sinus) or the “cord sign” (thrombosis of cortical or deep veins).5,10,11 In a contrast-enhanced head CT, the “empty delta sign” (non-opacified thrombus surrounded by the collateral veins of the sinus wall after injection of contrast) is consistent with a diagnosis of CVT but is only present 10-30% of the cases.5,10,11 Overall, the majority of non-contrast head CTs in CVT will be normal or have nonspecific signs of increased ICP, parenchymal abnormalities, or infarctions in multiple arterial distributions.4,5,10,11 CT venography has an overall sensitivity of 95% for CVT and is more readily available in most EDs than MRI.10 However, concerns of radiation exposure may limit its use in the younger population. MRI findings alone are dependent on the age of thrombus and thus venography is required.10 MRV is the most sensitive test and is the gold standard for diagnosing CVT.10,11


Why we miss it:
CVT is a Rare Disease
As mentioned, the incidence of CVT is quite low, ranging from 0.3-1.5 cases/100,000 person-years.3,4 One of the largest observational studies to date, the VENOST Study, captured 1144 cases from 2000-2015, at 35 large stroke centers in Turkey.12
The characteristics of headache associated with CVT are inconsistent… if they are present
As headaches are a common chief complaint in the ED, diagnosing CVT can be like finding a needle in a haystack. Studies have found headache to be the most common presenting complaint of CVT, occurring 62-95% of the time.11,12 However this means that 5% or more of cases will occur without a headache.11 Additionally, while neurological abnormalities are often taught as a trigger for additional evaluation in patients presenting with headache, the VENOST study found that 25% of cases of CVT presented as isolated headache.12 Unlike with subarachnoid hemorrhage, the headache in CVT tends to be more subacute.5,11,12 One study found that 57% of patients presented with subacute and chronic headaches, present anywhere from 4 days to greater than 2 weeks, leaving 43% presenting acutely.12 Another study reported 60% of patients presented acutely, although this study defined acute as 1-4 days after symptom onset.5 While rare, there have been case reports of CVT presenting as maximal in onset, “thunderclap” headaches, which could prove problematic as clinicians may anchor on ruling out a subarachnoid hemorrhage.5
Associated signs and symptoms are nonspecific
Focal neurological deficits, including cranial nerve palsies and visual field defects, occur in 30-50% of cases of CVT.4,5,13 Studies have reported aphasia and other focal cortical functions, like extremity weakness, as presenting symptoms, although this seems to be less common, occurring in only 2-3% of cases.3,5,10 Papilledema is seen in 30-60% of patients with CVT.3 More often, if there is extensive cortical involvement, the patient may present with altered mental status or encephalopathy, which occurs in around 17-20% of cases.10 Seizures occur in 24-40% of patients with CVT, which is markedly higher than in those patients with arterial ischemic stroke, where seizures occur in 2-9%.3,4,5,10,11,12 Thus CVT are an important diagnostic consideration in patients presenting with seizure and a focal neurologic deficit.
Headache that is worsened with Valsalva can be suggestive of CVT; however this is a non-specific finding, being a common feature in other diseases that present with headache and increased intracranial pressure.5 The location of headache in CVT is variable given the various locations of the venous sinuses, although it is questionable if location of thrombus will necessarily produce headache on the same side.3 Headaches can be unilateral, bilateral, frontal, or occipital.3,11 A study looking at the clinical features of CVT found that headache plus papilledema and headache plus seizure both had a positive predictive value of 0.57, and a specificity of 97-99% however the sensitivity was only 7-10%.13 Unfortunately no combination of two features had high enough positive predictive or negative predictive values to rule in or rule out the diagnosis.13
Risk factors are helpful but can be overlooked or unknown at the time of presentation
Common risk factors for CVT include OCP use and pregnancy or the peripartum period.4 Estimates of OCP use in CVT vary widely, possibly related to different rates of OCP use in different populations. One study estimated OCP use among 54-71% of female patients with CVT, while another found OCP use in 10-73% of female patients with CVT.4,5,8,12 Pregnancy and the first 6 weeks post-partum are higher risk periods for CVT. One study estimated an odds ratio (OR) of 17.24 for pregnancy/post-partum period for CVT, with a 95% confidence interval of 6.83-44.04.8 While this is a wide confidence interval, it is clear that CVT should be considered as a potential diagnosis in headache presentations this population. However, it may be easy to miss that a patient is post-partum if the clinician does not specifically inquire regarding recent pregnancy or delivery. OCP use can also be easily missed if a thorough medication review is not performed. Additional risk factors identified for CVT include glucocorticoid use (OR=18.26; 95% CI 3.25-102.55) and antiphospholipid syndrome (OR 6.98; 95% CI 2.06-23.6).8 Although knowledge of patient genetic risk factors might not be readily available in the ED, they are important to know as they place patients at greater risk for CVT. Some important genetic predispositions include factor V Leiden (OR 2.51; 95% CI 1.93-3.27), prothrombin 20210A (OR 5.53; 95% CI 3.98-7.69), and protein C deficiency (OR 10.74; 95% CI 3.07-37.65).8 Although many of these risk factors have useful ORs, their presence may not be known at the time of diagnosis.
CVT mimics are more common diagnoses
CVT can mimic more common diagnoses, causing clinicians to fall prey to premature closure. In order to understand why this diagnosis is commonly missed, it is important to recognize the situations where clinicians fail to consider this diagnosis and as well as what other diagnoses might mimic CVT. Additionally, as this diagnosis is made radiologically, it will be important to consider that it may be missed if physicians 1) do not order an initial head CT 2) Rely on a negative noncontrast head CT to exclude it, and 3) do not proceed to a CT venogram or MRI/MRV with non-specific or normal initial head CT.
Headache will be the most common scenario when CVT should be considered as a diagnosis. While primary headache disorders, such as tension-type headaches and migraines, will account for the overwhelming majority of headaches seen in the emergency department, it is important to know when to consider headache as a symptom of another, often more insidious, disease process.2,14 A headache that lacks any of the findings or patient risk factors that discussed above often do not require an extensive evaluation.2 While CVT can present as an isolated headache, inclusion of the diagnosis in the differential of any headache presentation and recognition of its risk factors can help decrease the risk of delayed or missed diagnosis. Therefore, it is necessary to review what additional features or patient risk factors warrant following a different diagnostic pathway:
- Meningitis: Headache with fever and/or neck stiffness should prompt evaluation for meningitis. Although this classic triad will only be present in 44% of patients with meningitis, the majority will have one or two symptoms.2 Meningeal signs and symptoms, like neck stiffness or nuchal rigidity should lead one towards meningitis, while focal neurological deficits and papilledema should raise suspicion for CVT.
- Subarachnoid hemorrhage (SAH): Headache, nausea/vomiting, neurological deficits, and seizures can all be presenting symptoms of SAH, making distinguishing this diagnosis from CVT challenging.2,14 SAH more commonly presents abruptly, with the headache reaching maximal intensity within a few minutes of onset, as opposed to headaches associated with CVT that typically develop over the course of days. Although both can present as a thunderclap headache, it is more common in SAH.2 SAH also tends to occur in a slightly older patient population, with an average age 57.15 The initial work up for both will require a head CT. Depending on the timing of patient presentation from the onset of headache, a lumbar puncture (LP) may also be necessary. If these two steps are non-diagnostic for SAH, in a patient with right risk factors, CVT should also be considered.
- Acute angle closure glaucoma: Headache, nausea/vomiting, and visual symptoms are common to both acute angle closure glaucoma and CVT.2 Abrupt onset, minutes to hours, rather than days, injected conjunctivae, blurry vision and decreased pupillary reactivity should lead one to suspect acute angle closure glaucoma over CVT.
- Carotid/vertebral artery dissection: Presenting as headache or neck pain with or without associated with focal neurologic deficits, a carotid or vertebral artery dissection is an important cause of secondary headache. Notably, like CVT, these conditions, can also present as an isolated headache.2 A history of trauma, neck manipulation, and sudden acceleration-deceleration should raise suspicion for vertebral or carotid dissection as the cause of a headache or neck pain. Horner’s syndrome, pulsatile tinnitus, and posterior circulation symptoms such as ataxia should also raise suspicion for dissection.2 If history and risk factors do not help distinguish this from CVT, the diagnosis of carotid or vertebral dissection requires CTA or MRA studies. While the timing of contrast administration for CVT differs from the timing for arterial studies to diagnose carotid or vertebral dissection, these imaging studies may still help clinicians work through and rule out secondary causes of headaches.
- Giant cell arteritis (GCA): Like in CVT, headache and visual symptoms can be the presenting complaints of GCA. Additional features such as jaw claudication and fever can help distinguish it from CVT.2 Additionally, GCA typically occurs in a much older patient population, with the majority of GCA diagnoses occurring in patients in their 70s, compared to the majority of CVT occurring in patients 20-35 year of age.2,12
- Idiopathic intracranial hypertension (IIH): Distinguishing IIH from CVT presents a diagnostic dilemma as they occur in similar patient populations and can both present with headache and cranial nerve palsies.16,17 Papilledema may be found on exam with both.2,14 The neurologic deficits associated with IIH are more commonly abducens nerve palsy and visual field defects.16 Other neurologic deficits in a patient with suspected IIH should also raise suspicion for CVT as the diagnosis. In a small study comparing MRI/MRV in patients with IIH and CVT, the IIH group was more likely to have signs of increased ICP on imaging, like flattening of the globe or an empty sella.16 An additional study found signs of increased ICP like peri-optic CSF and optic nerve tortuosity to be more common in IIH.17 These studies were conducted by trained neuroradiologists and thus have limited generalizability to the ED.
- Preeclampsia/eclampsia: This again presents a diagnostic dilemma to the emergency clinician who is taking care of a pregnant or postpartum patient with a headache, visual complaints, and/or nausea and vomiting.2 If there are other signs of preeclampsia, such a hypertension or laboratory signs of end organ damage such as proteinuria, clinicians should consider and rule out preeclampsia as a cause of the patient’s symptoms. The presence of focal neurological deficits should raise suspicion for CVT. If the patient begins to have a seizure, they should be treated as eclampsia until proven otherwise.3
Laboratory tests are not helpful
Laboratory evaluation is generally not useful in the evaluation of CVT. D-dimer is classically thought to be elevated in CVT, but the false negative rate is thought to be anywhere from 24-40%.1,3,4,5,11 Additionally given the many other conditions that can elevate D-dimer, this test cannot be relied on to rule in or out a diagnosis of CVT.11 A review by the European Stroke Organization found that D-dimer was more likely to be falsely negative in CVT patients who presented with headache alone.18 They also reported no difference in D-dimer levels in CVT patients with and without focal neurologic deficits.18 The European Stroke Organization found that thrombophilia screening did not aid in the diagnosis or functional outcome of CVT patients and thus recommend against routine screening in patients suspected of CVT.18
Lumbar puncture findings are similarly non-specific in CVT.10 Increased opening pressure, pleocytosis, and increased red blood cells may all be found.10 A study of LP in CVT patients found that LP was normal in 44% of patients with CVT.19 This study also found that the performance of an LP did not seem to affect the prognosis of patients with CVT.19 However, particularly among acutely ill patients, LP will be essential in ruling out other life threatening diagnoses like bacterial meningitis.
How we can improve
Consider the diagnosis
Failure to consider the diagnosis will inevitably lead to failure to diagnose. As this is a rare disease that can mimic more common conditions, clinicians should be aware of high risk populations and red flags on history and physical examination so as to identify the patient with a headache that requires further evaluation for CVT. Clinicians must also continue to consider other life-threatening causes of headache such as SAH.
Remember the risk factors
As discussed above, 85% of patients with CVT will have at least one identifiable risk factor.8,9 OCP use, pregnancy/peripartum status, and hypercoagulability are some of the most important risk factors.8 Another important potential risk factor is recent neurosurgery. A patient who had recent neurologic surgery who then presents to the ED with a headache, especially with a focal neuro deficit, should receive neurosurgical consultation to determine the etiology and if evaluation for CVT specifically is warranted.1,8 Clinicians can improve their sensitivity for this relatively rare diagnosis by taking thorough histories, checking medication lists, and considering imaging in patients presenting with headache and the risk factors discussed. Interestingly, in a study of risk factors for CVT, smoking, hypertension, and diabetes did not reach statistical significance, possibly reflecting the younger age of the population most affected by CVT. Thus, the emergency clinician should think about CVT in patients with stroke-like symptoms without these traditional stroke risk factors.8
Evaluate for signs of increased ICP in patients presenting with headache
Headache plus a symptom suggestive of increased ICP, such as blurry vision or morning nausea, could be a helpful framework for thinking about CVT.1,2,11,13 Clinicians should perform a thorough neurologic exam on all headache patients to assess for a focal neurological deficit. While fundoscopy can be challenging practice to incorporate into a busy ED environment, papilledema is an important physical exam finding of increased ICP. As previously discussed, headaches worsened by Valsalva and cranial nerve palsies are important diagnostic clues for CVT.5 The presence of a prothrombotic risk factor and clinical findings of increased ICP will be essential in guiding clinicians in the decision to evaluate for CVT.
Consider CVT in patients with non-headache presentations
Two other relatively common scenarios require consideration for CVT. While the patients presenting as below may require extensive diagnostic evaluations, the diagnosis of CVT may be missed if it is not considered.11
- The actively-seizing patient: While seizure control takes precedence over any diagnostic evaluation in these patients, when appropriate, consideration of risk factors for CVT and imaging evaluation may aid in making the diagnosis in these atypical presentations.
- Altered mental status: Patients presenting with lethargy or symptoms concerning for encephalopathy may require evaluation for CVT if other more common causes of encephalopathy are ruled out. Since CVT tends to occur in a younger population, this diagnosis should be considered in younger patients with risk factors and otherwise unexplained altered sensorium.
Treatment
Stabilization of the patient in the ED is the first priority. Actively seizing patients should be treated with benzodiazepines and other antiepileptics and their airways should be secured if necessary. Increased intracranial pressure can be treated with mannitol or hypertonic saline, particularly if herniation is a concern.1,4,5,11 The acute management of CVT itself is anticoagulation. In the acute phase, the patient should be started on heparin, even if the CVT is complicated by hemorrhagic transformation.11 The patient should be admitted to a stroke or neurology service for monitoring. Recent studies have shown long-term treatment with direct oral anticoagulants over low-molecular weight heparin is as effective, but the choice of agent and duration of anticoagulation should be made in consultation with neurology or hematology.4,11 Although there are case reports of mechanical thrombectomy, there is no clear evidence that this is effective, and transfer to a hospital with capabilities for neuro-endovascular thrombectomy is not mandatory.4 Older studies show a mortality of around 5%, although more recent studies estimate mortality around 2%.6 Eighty-five percent of patients who receive treatment with anticoagulants will show recanalization of their thrombosed vessel over time.4,5,6 Most patients will achieve complete recovery of their neurologic deficits, although chronic headaches can be a problem in a majority of patients for up to three years.4,5,6
Pearls and pitfalls
- CVT is a rare entity, and diagnosis will require consideration of this disease, as the associated headache can be highly variable, signs and symptoms are non-specific, and labs are not helpful in making the diagnosis.
- In the younger patient presenting with headache with focal neurologic deficits or a new stroke without traditional risk factors, consider CVT, particularly if they have important risk factors like peripartum status, OCP use, or history of hypercoagulability. 85% of patients with CVT will have at least one identifiable risk factor.
- Failure to consider the diagnosis, not investigating for risk factors, and not assessing for signs and symptoms of increased ICP will lead to missed diagnosis.
- In all patients presenting with headache, consider the secondary causes of headache, and which, if any, may need to be ruled out.
- A non-contrast head CT will only have specific signs of CVT 25% of the time and will be completely normal in 30% of CVT patients. If suspicion for the diagnosis is high, and the non-contrast study is normal, a CT venogram or MRV is necessary to rule out CVT.
References/Further Reading:
- Walls, Ron M., Hockberger, Robert S., Gausche-Hill, Marianne. Rosen’s Emergency Medicine: Concepts and Clinical Practice. Ninth Edition. Philadelphia, PA. Elsevier/Saunders, 2018: 153-159; 1273-1274.
- Tabatabai RR, Swadron SP. Headache in the emergency department: Avoiding misdiagnosis of dangerous secondary causes. Emergency medicine clinics of North America. 2016;34(4):695-716. https://www.ncbi.nlm.nih.gov/pubmed/27741984. doi: 10.1016/j.emc.2016.06.003.
- Kristoffersen ES, Harper CE, Vetvik KG, Faiz KW. Cerebral venous thrombosis – epidemiology, diagnosis and treatment. Cerebral venetrombose – forekomst, diagnostikk og behandling. Tidsskr Nor Laegeforen. 2018;138(12):10.4045/tidsskr.17.1047. Published 2018 Aug 20. doi:10.4045/tidsskr.17.1047
- Ferro J, Aguiar de Sousa D. Cerebral venous thrombosis: An update. Curr Neurol Neurosci Rep. 2019;19(10):1-9. https://search.proquest.com/docview/2277839622. doi: 10.1007/s11910-019-0988-x.
- Mehta A, Danesh J, Kuruvilla D. Cerebral venous thrombosis headache. Curr Pain Headache Rep. 2019;23(7):1-6. https://www.ncbi.nlm.nih.gov/pubmed/31147848. doi: 10.1007/s11916-019-0786-9.
- Coutinho JM, Zuurbier SM, Stam J. Declining mortality in cerebral venous thrombosis: a systematic review. Stroke. 2014;45(5):1338-1341. doi:10.1161/STROKEAHA.113.004666
- Hufnagle JJ, Tadi P. Neuroanatomy, Brain Veins. [Updated 2020 Jan 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546605/
- Green M, Styles T, Russell T, et al. Non-genetic and genetic risk factors for adult cerebral venous thrombosis. Thrombosis Research. 2018;169:15-22. http://dx.doi.org/10.1016/j.thromres.2018.07.005. doi: 10.1016/j.thromres.2018.07.005.
- Zimny A, Dziadkowiak E, Bladowska J, et al. Cerebral venous thrombosis as a diagnostic challenge: Clinical and radiological correlation based on the retrospective analysis of own cases. Advances in clinical and experimental medicine : official organ Wroclaw Medical University. 2017;26(7):1113-1122. https://www.ncbi.nlm.nih.gov/pubmed/29211360. doi: 10.17219/acem/66778.
- Mehndiratta MM, Garg S, Gurnani M. Cerebral venous thrombosis–clinical presentations. Journal of the Pakistan Medical Association. 2006;56(11):513. https://www.ncbi.nlm.nih.gov/pubmed/17183979.
- Long, B., Koyfman, A. “Cerebral Venous Sinus Thrombosis: Pearls and Pitfalls”. net. July 6th, 2016. http://www.emdocs.net/cerebral-venous-thrombosis-pearls-and-pitfalls/ Accessed: June 18, 2020
- Duman T, MD, Uluduz D, MD, Midi I, MD, et al. A multicenter study of 1144 patients with cerebral venous thrombosis: The VENOST study. Journal of Stroke and Cerebrovascular Diseases. 2017;26(8):1848-1857. https://www.clinicalkey.es/playcontent/1-s2.0-S1052305717301787. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.020.
- Tanislav C, Siekmann R, Sieweke N, et al. Cerebral vein thrombosis: Clinical manifestation and diagnosis. BMC neurology. 2011;11(1):69. https://www.ncbi.nlm.nih.gov/pubmed/21663613. doi: 10.1186/1471-2377-11-69.
- Do T, Remmers A, Schytz H, et al. Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology. 2018;92(3):134-144. https://www.ncbi.nlm.nih.gov/pubmed/30587518. doi: 10.1212/WNL.0000000000006697.
- Bian L, Liu Y, Nichols LT, et al. Epidemiology of subarachnoid hemorrhage, patterns of management, and outcomes in china: A Hospital‐Based multicenter prospective study. CNS Neuroscience & Therapeutics. 2012;18(11):895-902. https://onlinelibrary.wiley.com/doi/abs/10.1111/cns.12001. doi: 10.1111/cns.12001.
- Ridha MA, Saindane AM, Bruce BB, et al. MRI findings of elevated intracranial pressure in cerebral venous thrombosis versus idiopathic intracranial hypertension with transverse sinus stenosis. Neuroophthalmology. 2013;37(1):1-6. doi:10.3109/01658107.2012.738759
- Onder H, Kisbet T. Neuroimaging findings in patients with idiopathic intracranial hypertension and cerebral venous thrombosis, and their association with clinical features. Neurol Res. 2020;42(2):141-147. doi:10.1080/01616412.2019.1710408
- Ferro JM, Bousser MG, Canhão P, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis – Endorsed by the European Academy of Neurology. Eur Stroke J. 2017;2(3):195-221. doi:10.1177/239698731771936
- Canhão P, Abreu LF, Ferro JM, et al. Safety of lumbar puncture in patients with cerebral venous thrombosis. Eur J Neurol. 2013;20(7):1075-1080. doi:10.1111/ene.12136









2 thoughts on “Cerebral venous thrombosis: why we miss it and how we can improve”
Pingback: Trombosis de Seno venoso, actualización. | Academia Emergencias
good article ,very usefull