Droperidol Use in the Emergency Department – What’s Old is New Again
- Aug 1st, 2019
- David Cisewski
- categories:
Authors: David Cisewski, MD (@dhcisewski– EM Resident Physician, Icahn School of Medicine at Mount Sinai) // Edited by: Manpreet Singh, MD (@MPrizzleER), Alex Koyfman, MD (@EMHighAK), and Brit Long, MD (@long_brit)

Introduction
Earlier this year American Reagent announced the re-introduction of droperidol back into the US market. This is bringing an old favorite back to many EM docs and a novel tool for new residents and attendings who have never used this before. This article serves as a primer (or refresher) on a drug with an anticipated rapid utilization among ED’s nationwide.
Lets start with the basics…. what is droperidol?
Droperidol is a dopamine antagonist (D2-R antagonist) from the butyrophenone family, a class of medications commonly known for their antipsychotic effects (think haloperidol, benperidol). Originally developed in 1961 by Janssen Pharmaceuticals as an anesthetic adjuvant in the operative setting, it was approved in 1971 by the USFDA for nausea, vomiting, anxiety, and sedation.
Droperidol is often favored due to it’s high potency, rapid onset, and relatively short duration compared to other sedatives. Onset of action is biphasic, with initial effect observed at 3 to 10 minutes following intravenous or intramuscular administration and peak response at approximately 30 minutes (1). Research has shown that “absorption is so rapid that one would expect the response following droperidol IM administration to be almost equivalent to IV administration” (1). Duration of effect is approximately 2 to 4 hours, although reduced alertness may persist for up to 12 hours (2). Droperidol undergoes hepatic metabolism with inactive metabolites excreted via urine and feces. Droperidol is listed as Category C for use during pregnancy.
What is it used for in the emergency setting?
Over the last several decades a number of uses have ‘emerged’ in the emergency setting:
Agitation. New emergency trainees may have become accustomed to the combination haloperidol-midazolam (and occasionally diphenhydramine) for patients in acute agitation unresponsive to verbal de-escalation (aka, B-52, “five-and-two”). Droperidol, however, has been shown to have a more rapid onset and greater efficacy than haloperidol (5 mg droperidol vs 10 mg haloperidol) for patients with acute psychosis (3), as well as for patients with agitated behavior in the ED (4). The DORM Study (Droperidol OR Midazolam) showed that 10 mg droperidol IM was just as effective in reducing the duration of violent behavior as compared to 10 mg midazolam (20 min vs 24 min), required less additional sedation (33% vs 62%) and was associated with less respiratory depression among intoxicated agitated patients (5). Taylor, et al found the combination of 5mg midazolam-5mg droperidol was more effective in attaining adequate sedation at 10 minutes compared to droperidol or olanzapine alone (75% vs 50% vs 50%) (6). Chan et al, found that the addition of 5mg droperidol (or 5 mg olanzapine) to 2.5 – 5 mg aliquots of midazolam resulted in a statistically significant reduction in time to sedation versus midazolam alone (21 min vs 14 min vs 68 min) (7).

Chan, 2013 – IV droperidol or olanzapine as an adjunct to midazolam is effective and decreases the time to adequate sedation compared to midazolam alone (control).
Bottom line: For agitation, 5 mg droperidol + 2 – 5 mg midazolam IM (repeat 5 mg droperidol as needed).
Headaches. 2.5 mg IM droperidol every 30 minutes (max 7.5 mg) has been shown to achieve a ‘mild/none’ headache status in 88-100% of patients presenting with acute migraines (87% female study population) (8). Weaver, et al found that 2.5 mg droperidol IV resulted in an improved 50% pain reduction at 30 minutes versus 10 mg prochlorperazine IV (83% vs 72%) (9). Miner, et al found 5 mg IM droperidol to be more efficacious at alleviating acute headaches than prochlorperazine (as measure by those having at least a 50% reduction in headache at 60 minutes ; 90% vs 68%, p = 0.017) (10). In a dose-ranging study conducted by Silberstein, et al 2.75 mg droperidol IM was associated with a statistically significant headache relief in 87% of patients who presented with moderate to severe acute migraine (11). Complete pain-free response was also optimized at 2.75mg (49%) with both higher and lower doses showing less efficacy at preventing headache recurrence and increased side effects among higher doses (11).

Silberstein, 2003 – 2.75 mg droperidol (3rd column) was the optimal dose for complete headache relief at 2 hours (column 1 – 0.1mg; 2 – 2.75mg; 3 – 5.5 mg; 4 – 8.25mg).
Bottom line: For headaches, 2.5-2.75 mg droperidol IM or IV (as a second-line agent behind metoclopramide)
Nausea. Research by Braude, et al showed that 1.25 mg droperidol resulted in a higher percentage reduction in nausea compared to metoclopramide or prochlorperazine though with more akathisia (71% vs 25% vs 35%) among patients presenting to the ED for nausea (12). Fortney, et al showed that 1.25 mg droperidol IV was more effective in reducing the incidence of emesis in the first 2 hours post-operative compared to 4mg ondansetron (69% vs 62%, P < 0.05) (13).
Bottom line: For nausea, 1.25 mg droperidol IM or IV (if nausea + headache, 2.5-2.75mg IM or IV)
Vertigo. In 1969, research out of JAMA ENT showed that droperidol could successfully suppress nystagmus (and thus labyrinthine vestibular vertigo) in rabbits put through a spinning vortex (14). Correlating these findings to patients in the emergency setting, Irving, et al found that 2.5 mg droperidol IM resulted in relief of vertiginous symptoms in 86% of patients at 30 minutes post-administration (an additional 7% improved but required a rescue dose) (15). Further research by Irving, et al showed 2.5 mg droperidol IM as effective as 50 mg dimenhydrinate (Dramamine) IM for the reduction of acute vertigo symptoms (42% vs 43% of patients felt well enough to return home at 30 minutes (‘get up and go’ test) (16).

Yelnosky, 1964 – Droperidol first shown to successfully suppress nystagmus (and thus labyrinthine vestibular vertigo) in rabbits put through a spinning vortex.
Bottom line: For vertigo, 2.5 mg droperidol IM or IV
Analgesic Adjunct. Lo, et al showed that 50 mcg droperidol added to 1mg morphine q5min delievered through a PCA pump resulted in a total morphine administration reduction over a 72h post-operative (post-hysterectomy) period compared to morphine alone (33 mg vs 54 mg) (17).
Bottom line: Consider small aliquots of droperidol as an opioid-sparing analgesic adjunct for patients requiring repeat administration of analgesics (more data required for a definitive recommendation).
Sounds like a great drug….. where’s it been the last two decades?
Great question. This is slowly becoming a story lost to the previous generation. However, there are important points to be learned from the droperidol story that should be remembered as we develop an evidence-based practice.
From 1961 to 2000, droperidol became a staple of emergency departments across the globe. In 2000, 25 million doses of generic droperidol were sold worldwide (18) and by 2001, droperidol held more than 30% of the market share for antiemetics (19). But in 2001, despite 30 years of seemingly safe and effective droperidol use, the FDA released a black box warning regarding the QT prolonging effects of droperidol (at doses >2.5mg) based on 273 cases of adverse effects as reported to MedWatch, claiming a concern for “reports of deaths associated with QT prolongation and torsade de pointes in patients treated with doses of Inapsine (droperidol) above, within and even below the approved range.” Essentially, droperidol could prolong QT, there was lots of risk factors to consider, ECG’s should be performed before, during, and after droperidol use, and if you chose to still use it, you would have the threat of a black box warning looming over you should anything go wrong.

FDA Black Box Warning on Droperidol (2001).
Seemingly overnight, the use of droperidol in the United States declined due to risk of legal implications (20). Hospital pharmacies dropped droperidol from the formulary and the 1.25 mg droperidol ($2.50) standard for nausea was replaced with on-patent 4 mg IV ondansetron (Zofran – $150) as the antiemetic of choice (21).
Red flags went up across the medical community resulting in a number of admirable independent reviews of the FDA data from which this warning was based upon (19, 22-24). One such review pointed out that 83% of the cases of QT prolongation were received from nations outside the United States with 49% of fatalities from droperidol occurring at doses of 50 mg or greater (up to 150mg)– multitudes higher than our typical dosing of 5 to 10 mg (25). Additionally, of the 273 cases that made up the black box warning, only 94 were unique cases identified as ‘associated’ with droperidol use (the rest duplicates) (22, 23). Further probing noted that 71 of the cases were submitted to MedWatch on a single day in 2001 despite the FDA regulations requiring pharmaceutical producers to report adverse events within 15 days of their becoming aware (22). It was highlighted that this release from Janssen-Cilag (the maker of on-patent risperidone) came just 3 months after it stopped selling droperidol as an antipsychotic in Europe (25). Was this a coincidence? What was to gain by the FDA warning? This is for you to decide.
Does droperidol really prolong QT and cause torsade de pointes?
The short answer is yes – droperidol does prolong the QT interval and a prolonged QT can lead to torsade de pointe. The 2019 American Reagent package insert continues to have the Black Box warning indicating, “all patients should undergo a 12-lead ECG prior to administration of droperidol to determine if a prolonged QT interval (i.e., QTc greater than 440 msec for males or 450 msec for females) is present. If there is a prolonged QT interval, droperidol should NOT be administered”.
However, data suggests QT prolongation is minimal and complications rare among droperidol use. Guy, et al showed that 0.25 mg/kg droperidol administered before elective surgery resulted in an average clinically significant increase of 24 milliseconds (p<.001) without episodes of torsade de pointes (26). Additionally, Lischke, et al conducted a prospective randomized double-blind control trial assessing dose-dependent QT prolongation droperidol (27). Doses of 0.1 mg/kg, 0.175 mg/kg, and 0.25 mg/kg (approximately 7mg, 12mg, 17.5 mg) were randomly administered with QT monitoring every minute over a 10 minute period. In each group, the maximum QTc interval prolongations occurred within 60 seconds of injection and did not further increase with time. The QTc intervals were noted to be larger with increasing the dose of droperidol. No patients experience torsade de pointes, even at these higher than standard doses.

Lischke, 1994 – QT prolongation dose-finding study. QT prolongation increased with increasing doses of droperidol administration with peak QT occurring within the first minute in each group.
So how long is too long? Although a QT interval of at least 500 milliseconds generally has been shown to correlate with a higher risk of torsade de pointes, there is no established threshold below which prolongation of the QT interval is considered free of pro-arrhythmic risk (28). Some experts have suggested that the “QT dispersion” (difference between the shortest and longest QT interval on a 12-lead ECG) that is a better marker of conduction instability with a dispersion >100 milliseconds resulting in an increased risk of torsades de pointes (29).

QT Dispersion as a marker of cardiac instability. A difference of >100ms between the shortest and longest QT interval on an ECG is associated with increased cardiac instability (Courtesy of Lederman, et al, 2014).
It’s important to note that the large psychiatric doses cited in the FDA warning (up to 150 mg) were substantially larger than anything we would use in the emergency setting. In one of the most solidifying studies conducted on droperidol of agitation, Calver, et al demonstrated that 10 mg droperidol given among a large cohort of 1,009 agitated patients (with repeat dosing at 15 min as needed) resulted in only 13 cases of prolonged QT (7 of which had prior reasons for QT prolongation) and no cases of torsade de pointes (30).
Interestingly, research has shown there this is no evidence to suggest that serotonin type 3-receptor (5-HT(3)) antagonists (eg, ondansetron) are safer than droperidol with respect to Q-T interval prolongation (19). This was further supported by Charbit, et al who found both droperidol and ondansetron induced similar clinically relevant QTc interval prolongation (31).
A decade after the droperidol black box warning, the FDA released a safety communication that ondansetron “may” be associated with abnormal heart rhythms. However, a safety communication is a far cry from a black box warning, a label that has yet to be lifted from droperidol.
Bottom line: Droperidol can result in QT prolongation with torsade de pointes being a rare complication among patients with normal QT intervals. “QT dispersion” may be considered a better indication of cardiac instability.
What else should I worry about with QT prolongation?
There are a lot of drugs and conditions that can prolong QT. It is advised to review the patient’s medication list, past medical history, and any other red flags that may support an underlying prolonged QT. The following is a list of medications and conditions that prolong QT (adaption of conditions listed from Electrocardiography In Emergency, Acute, and Critical Care (32)).

List of medications and conditions that prolong QT (adaption of conditions listed from Electrocardiography In Emergency, Acute, and Critical Care (32)).
So should we always be getting an ECG on these patients before droperidol use?
In the acutely agitated patient this may not be feasible. Based on the results from Lischke, et al showing that QT max occurs at approximately 60 seconds, a post-administration ECG may be too late to detect an increased risk of QT prolongation. It is important to consider the patient and the potential risk factors for prolonged QT prior to administration. Research has shown that female sex, geriatric patients (>65yo), known prolonged QT, history cardiovascular disease or congestive heart failure, bradycardia, electrolyte abnormalities (hypomagnesemia, hypocalcemia, hypokalemia), history of cardioversion are individually associated with a prolonged QT (33). If time allows, consider calculating a Tisdale Risk Score to assess the risk of prolonged QT. Though not terribly sensitive or specific it does get you thinking about the potential risk factors.

Tisdale Risk Score. Risk stratification tool used to assessment patient at low, medium, and severe risk of prolong-QT.
When in doubt, get the ECG before the first dose and prior to a repeat dose whenever possible. Any patients with a cardiovascular risk factors or one other QT-prolonging medications should receive a baseline ECG (19):
- Q-Tc interval of <410ms = low risk à limited risk to medication use
- Q-Tc interval of 420-440ms = moderate risk à give cautiously, post-admin ECG
- Q-Tc interval of ≥450 ms = high risk à consider alternatives
Bottom line: When in doubt (and when possible), get an ECG.
I’m in….. anything I need to worry about when using it?
When administering droperidol, always be aware of the side effects and warning signs of acute toxicity. Common side effects include akathisia, dystonia, sedation, neuroleptic malignant syndrome (lead-pipe rigidity, hyperthermia, autonomic instability, and cardiovascular collapse). Hypotension is an uncommon but potential side effect and as such droperidol should not be used among hemodynamically unstable patients. Whenever possible, have the patient on a cardiac monitor prior to administration. Last but not least have the magnesium on hand if those ‘points start twisting’.
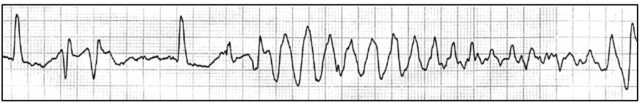
Torsade de Pointes (‘twisting of the points’). A potential complication of QT prolongation.
So the data is in….. where do we stand now?
A 2015 American Academy of Emergency Medicine (AAEM) Position statement made the following statement of the safety of droperidol:
“Droperidol is an effective and safe medication in the treatment of nausea, headache, and agitation. The literature search did not support mandating an electrocardiogram or telemetry monitoring for doses < 2.5 mg given either IM or IV. IM doses of up to 10 mg of droperidol seem to be as safe and as effective as other medications used for sedation of agitated patient.”
But allowance and accessibility were not yet matched until 2019 with the re-introduction of droperidol by American Reagent bringing the drug back into ED’s nationwide. So for now, droperidol is back and we have a substantial body of evidence to indicate it is safe and effective for use among a wide range of patients in the ED setting.
Main Takeaways on Droperidol in the Emergency Setting:
- Droperidol is back and its safe
- Onset – 5 to 10 minutes; Duration – 2 to 4 hours
- Potential uses for droperidol
- Agitation
- Headache
- Nausea
- Vertigo
- Opioid Adjunct
- Review QT prolonging risk factors prior to use; when in doubt, get an ECG
- Consider the “QT dispersion” (difference between the shortest and longest QT interval on a 12-lead ECG) as a marker of conduction instability (>100 milliseconds resulting in an increased risk of torsades de pointes)
- Watch for side effects: akathisia, dystonia, sedation, neuroleptic malignant syndrome (lead-pipe rigidity, hyperthermia, autonomic instability, and cardiovascular collapse), hypotension
Further Reading:
- Reuben Strayer @ SMACC – Disruption, Danger And Droperidol- Emergency Management Of The Agitated Patient
- Newman, 2015 – Training the Mind, and the Food and Drug Administration, on Droperidol (24)
- EP Monthly: Droperidol Gets Dropped
References:
[1] Cressman WA, Plostnieks J, Johnson PC. Absorption, metabolism and excretion of droperidol by human subjects following intramuscular and intravenous administration. Anesthesiology. 1973;38:363-9.
[2] Hospira. Droperidol Package Insert. http://labeling.pfizer.com/ShowLabeling.aspx?id=4412.
[3] Resnick M, Burton BT. Droperidol vs. haloperidol in the initial management of acutely agitated patients. J Clin Psychiatry. 1984;45:298-9.
[4] Thomas H, Jr., Schwartz E, Petrilli R. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Ann Emerg Med. 1992;21:407-13.
[5] Isbister GK, Calver LA, Page CB, Stokes B, Bryant JL, Downes MA. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56:392-401 e1.
[6] Taylor DM, Yap CYL, Knott JC, et al. Midazolam-Droperidol, Droperidol, or Olanzapine for Acute Agitation: A Randomized Clinical Trial. Ann Emerg Med. 2017;69:318-26 e1.
[7] Chan EW, Taylor DM, Knott JC, Phillips GA, Castle DJ, Kong DC. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61:72-81.
[8] Wang SJ, Silberstein SD, Young WB. Droperidol treatment of status migrainosus and refractory migraine. Headache. 1997;37:377-82.
[9] Weaver CS, Jones JB, Chisholm CD, et al. Droperidol vs prochlorperazine for the treatment of acute headache. J Emerg Med. 2004;26:145-50.
[10] Miner JR, Fish SJ, Smith SW, Biros MH. Droperidol vs. prochlorperazine for benign headaches in the emergency department. Acad Emerg Med. 2001;8:873-9.
[11] Silberstein SD, Young WB, Mendizabal JE, Rothrock JF, Alam AS. Acute migraine treatment with droperidol: A randomized, double-blind, placebo-controlled trial. Neurology. 2003;60:315-21.
[12] Braude D, Soliz T, Crandall C, Hendey G, Andrews J, Weichenthal L. Antiemetics in the ED: a randomized controlled trial comparing 3 common agents. Am J Emerg Med. 2006;24:177-82.
[13] Fortney JT, Gan TJ, Graczyk S, et al. A comparison of the efficacy, safety, and patient satisfaction of ondansetron versus droperidol as antiemetics for elective outpatient surgical procedures. S3A-409 and S3A-410 Study Groups. Anesth Analg. 1998;86:731-8.
[14] Harris I, Eviatar A, Goodhill V. Droperidol and fentanyl citrate compound as a vestibular depressant. Arch Otolaryngol. 1969;89:482-7.
[15] Irving C, Richman PB, Kaiafas C, Eskin B, Ritter A, Allegra J. Droperidol for the treatment of acute peripheral vertigo. Am J Emerg Med. 1999;17:109-10.
[16] Irving C, Richman P, Kaiafas C, Eskin B, Allegra J. Intramuscular droperidol versus intramuscular dimenhydrinate for the treatment of acute peripheral vertigo in the emergency department: a randomized clinical trial. Acad Emerg Med. 2002;9:650-3.
[17] Lo Y, Chia YY, Liu K, Ko NH. Morphine sparing with droperidol in patient-controlled analgesia. J Clin Anesth. 2005;17:271-5.
[18] White PF. Droperidol: a cost-effective antiemetic for over thirty years. Anesth Analg. 2002;95:789-90.
[19] Jackson CW, Sheehan AH, Reddan JG. Evidence-based review of the black-box warning for droperidol. Am J Health Syst Pharm. 2007;64:1174-86.
[20] Young D. Black-box warning for droperidol surprises pharmacists. Am J Health Syst Pharm. 2002;59:494, 7, 502-4.
[21] Gan TJ. “Black box” warning on droperidol: report of the FDA convened expert panel. Anesth Analg. 2004;98:1809.
[22] Horowitz BZ, Bizovi K, Moreno R. Droperidol–behind the black box warning. Acad Emerg Med. 2002;9:615-8.
[23] Kao LW, Kirk MA, Evers SJ, Rosenfeld SH. Droperidol, QT prolongation, and sudden death: what is the evidence? Ann Emerg Med. 2003;41:546-58.
[24] Newman DH. Training the Mind, and the Food and Drug Administration, on Droperidol. Ann Emerg Med. 2015;66:243-5.
[25] van Zwieten K, Mullins ME, Jang T. Droperidol and the black box warning. Ann Emerg Med. 2004;43:139-40.
[26] Guy JM, Andre-Fouet X, Porte J, Bertrand M, Lamaud M, Verneyre H. [Torsades de pointes and prolongation of the duration of QT interval after injection of droperidol]. Ann Cardiol Angeiol (Paris). 1991;40:541-5.
[27] Lischke V, Behne M, Doelken P, Schledt U, Probst S, Vettermann J. Droperidol causes a dose-dependent prolongation of the QT interval. Anesth Analg. 1994;79:983-6.
[28] Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA. 2003;289:2120-7.
[29] Uyarel H, Ozdol C, Gencer AM, Okmen E, Cam N. Acute alcohol intake and QT dispersion in healthy subjects. J Stud Alcohol. 2005;66:555-8.
[30] Calver L, Page CB, Downes MA, et al. The Safety and Effectiveness of Droperidol for Sedation of Acute Behavioral Disturbance in the Emergency Department. Ann Emerg Med. 2015;66:230-8 e1.
[31] Charbit B, Albaladejo P, Funck-Brentano C, Legrand M, Samain E, Marty J. Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology. 2005;102:1094-100.
[32] Mattu A, Tabas J, Brady W. Electrocardiography In Emergency, Acute, And Critical Care: American College of Emergency Physicians; 2019.
[33] Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013-22.

One thought on “Droperidol Use in the Emergency Department – What’s Old is New Again”