We always work hard, but we may not have time to read through a bunch of journals. It’s time to learn smarter.
Originally published at JournalFeed, a site that provides daily or weekly literature updates.
Follow Dr. Clay Smith at @spoonfedEM, and sign up for email updates here.
#1: Extracorporeal Life Support in the Emergency Department
What is ECLS?
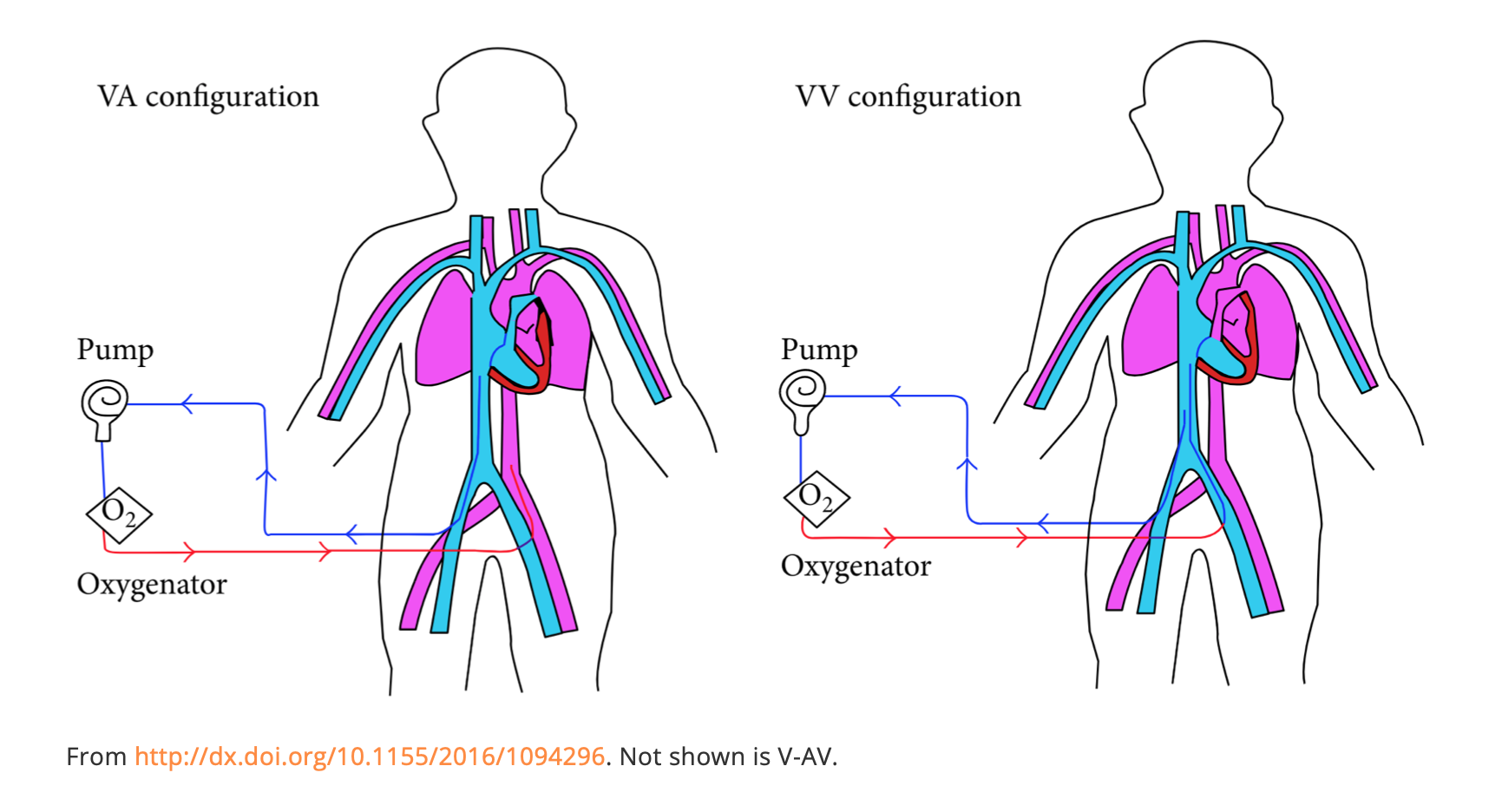
Extracorporeal life support (ECLS, aka ECMO) in its simplest form is taking out a patient’s blood, sending it through a pump and membrane to oxygenate and remove CO2, and putting it back in. There are two main types: veno-venous (V-V) and veno-arterial (V-A).
- V-V is most common and is used for inability to oxygenate despite maximal ventilator settings in the absence of cardiovascular collapse. In V-V, blood is sucked out of a femoral vein (FV) or internal jugular vein (IJ), sent through the pump to a membrane to remove CO2 and add oxygen, and returned to the right atrium (RA). It is returning oxygenated blood to the RA since the lungs aren’t working. There are single cannula systems via the IJ with a proximal port in the RA and distal in the IVC. Flow rate is up to 6L/min.
- V-A is used for situations with poor perfusion and need for circulatory support. In V-A, a FV cannula sucks blood out, into the pump, to the membrane for oxygenation and removal of CO2, and returns it to the femoral artery (FA). It can provide up to 10L/min flow.
- There is also a hybrid model, V-AV for severely compromised cardiac and pulmonary function. With these, blood is sucked out of the IVC, oxygenated, and pumped back to both the RA (for oxygenation) and FA (for circulatory support).
Newer cannulas, better pumps, and miniaturized ECLS devices, have made this heroic procedure more accessible and feasible for use in the ED.
Why Do ECLS?
It should be used in conditions causing cardiac or pulmonary failure that are reversible. It can buy time for diagnostics or procedures. ECLS is estimated to be appropriate in about 2.5 patients per 10,000 ED visits.
ECLS is useful as a temporary bridge for potentially reversible conditions for which a definitive fix is available or possible. Survival with good neurological outcome is better in select patients than those with standard CPR.
V-A ECLS
- Arrest needing PCI
- MI with shock
- Aortic dissection with tamponade
- Sepsis related cardiac dysfunction
- Hypothermia with refractory VT
- Bridge to damage control surgery/procedure
- PE with CV collapse
- Other refractory shock or arrest
- Cardiodepressant overdose
V-V ECLS
- Respiratory failure
- Drowning
- Amniotic fluid/fat embolism
- Air embolism
V-AV ECLS
- Cardiogenic shock with severely compromised lung function, i.e. pumonary edema/ARDS
Contraindications
- Prolonged or unwitnessed arrest with brain injury
- Other end stage illness or condition incompatible with life
- Intracranial bleed or irreversible brain injury
- Uncontrolled hemorrhage
- Advanced age (relative contraindication)
- Anatomic issues with cannula sites
Who’s Involved?
This requires a team of nurses, RTs, and physicians with specialized training in ECLS and the ability to troubleshoot and manage sudden circuit failure.
What Could Possibly Go Wrong?
These are quoted directly from the cited article, Table 2.
Cannulation-related
- Failure to complete cannulation
- Major vessel perforation or dissection
- Cannulation of the wrong vessel
- Pseudoaneurysm
- Retroperitoneal hemorrhage
- Cannula malposition or migration
- Distal limb ischemia
- Accidental decannulation
- Cannula clotting
Circuit-related
- Hemolysis
- Pump or oxygenator malfunction or failure
- Inadequate drainage
Hemorrhagic
- Cannula site bleeding
- Intracerebral hemorrhage
- Retroperitoneal hemorrhage
- Gastrointestinal hemorrhage
- Pulmonary hemorrhage
- Systemic fibrinolysis and coagulopathy
Other
- Thromboembolism (arterial or venous)
- Hypoperfusion (end-organ injury including multiple organ failure)
- LV distension and pulmonary edema (VA support)
- Cannula site infection
- Cannula-related bloodstream infection
Logistics for Transport
- Trained ECLS team members
- Backup circuit components pre-primed
- Back up batteries/fully charged power sources
- Secure all cannulas
- Transport pack: “clamps, scissors, gloves, fluids, hand ventilation bag, monitor, ventilator, small procedure tray, chest tubes”
- Full O2 tank(s)
- Emergency drugs, IV pumps
Another Spoonful
- LITFL has the quickest, simplest summary of ECLS.
- There is an entire website called edecmo.org.
- And the Reanimate Conference focuses on cutting edge resuscitation topics, such as ECLS.
Source
Extracorporeal life support in the emergency department: A narrative review for the emergency physician. Resuscitation. 2018 Dec;133:108-117. doi: 10.1016/j.resuscitation.2018.10.014. Epub 2018 Oct 15.
Open in Read by QxMD
#2: Top 10 Signs Your Cancer Patient May Become Critically Ill
Spoon Feed
This top 10 list may be life saving by helping us identify which cancer patients have a greater chance of becoming critically ill.
Why does this matter?
Patients with cancer are at greater risk of complications and have higher mortality. What are some other clues that may indicate greater illness severity in cancer patients?
Top 10: cancer, cancer, cancer, cancer…
The authors compiled a top 10 list of signs that may indicate that the patient with cancer in your ED is at greater risk of becoming critically ill.
- Ignorance is not bliss. Knowing the, “underlying malignancy, comorbidities, autonomy, history of fungal or multiresistant bacterial infection,” is crucial. The patient or family is unlikely to know this key information.
- Performance status – When you don’t have all the key information, yet have to make disposition decisions, consider calculating performance status (thanks MDCalc!). Poor performance status increases risk of critical illness.
- Level of immunodepression – Be especially wary in patients with allogeneic bone marrow transplant, prolonged (>7 days), or profound neutropenia (< 100/mm3), as these can result in severe infection and increase risk of fungal disease. Immune checkpoint inhibitors cause immune enhancement but still may have profound toxicity.
- Pulmonary involvement – Dyspnea from pneumonia, presence of hypoxia, or dyspnea from anemia, PE, heart failure, or chemo toxicity to heart or lung, are all markers of severe illness.
- Sepsis – Cancer patients with sepsis need ICU care. Neutropenia alone doesn’t – unless septic.
- Metabolic disturbance – Hyperkalemia, hyperphosphatemia, and hyperuricemia may all accompany tumor lysis. Induction chemo for some malignancies is often best started in the ICU. Don’t forget about the metabolic dangers from hyperglycemia, adrenal insufficiency, or hypercalcemia related to cancer.
- Coagulation – DIC may accompany some promyelocytic leukemias. Hypercoagulability and venous thromboembolism is an ever-present risk in cancer patients.
- Neurological signs – Tumor infiltration, edema, leptomeningeal spread, infection, or bleeding may all cause neurological emergencies. Emergent MRI or LP may be needed for some.
- Absence of clear diagnosis – Don’t blow off cancer patients or skimp on the workup. Err on the side of being more thorough. Failing to find reversible causes of illness may cause rapid decompensation.
- Delayed ICU admission – Many of our illness severity scoring systems don’t apply to cancer patients. Have a lower threshold to admit to the ICU if they have abnormal vital signs.
Source
The 10 signs telling me that my cancer patient in the emergency department is at high risk of becoming critically ill. Intensive Care Med. 2018 Dec;44(12):2315-2318. doi: 10.1007/s00134-018-5449-5. Epub 2018 Nov 12.
Open in Read by QxMD
#3: AAP – No More Hypotonic MIVF
Spoon Feed
The AAP now recommends isotonic fluid (+/- KCl and dextrose) in children >28 days to <18 years who need maintenance IV fluid, as opposed to hypotonic solutions.
Why does this matter?
For years (really since the 1950s) hypotonic solutions been recommend for use in children to meet maintenance fluid requirements. This AAP update was much needed.
Big change
This is an AAP Clinical Practice Guideline. Hyponatremia and its associated complications of encephalopathy or edema occur with regularity. Hypotonic fluid increases the risk of hyponatremia. So, the AAP convened a group of experts and performed a comprehensive literature review. They came up with this statement for children requiring maintenance IV fluid.
“The AAP recommends that patients 28 days to 18 years of age requiring maintenance IVFs should receive isotonic solutions with appropriate potassium chloride (KCl) and dextrose because they significantly decrease the risk of developing hyponatremia.” This was based on high quality, strong evidence.
Exclusions were: “Patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, or severe burns; neonates who are <28 d old or in the NICU; or adolescents >18 y old.”
They comment on the concern for hyperchloremic metabolic acidosis. This is indeed a concern. Recall that in critically ill children hyperchloremia was associated with increased mortality. They plan to address the issue of lower chloride, balanced solutions in future guidelines.
Source
Clinical Practice Guideline: Maintenance Intravenous Fluids in Children. Pediatrics. 2018 Dec;142(6). pii: e20183083. doi: 10.1542/peds.2018-3083.
Open in Read by QxMD
#4: Hyper-K Treatment REVEAL-ED
Spoon Feed
Medical therapy for hyperkalemia is highly variable and dropped the level by about 1mmol/L in 4 hours. Dialysis dropped it about 2.2mmol/L in 4 hours.
Why does this matter?
Hyperkalemia is a potentially fatal electrolyte abnormality. We need to be expert in treating it. This is a look at how it was done across multiple EDs and how effective it was in lowering potassium. Or you could skip this and listen to Dr. Rob Orman’s ERcast on hyper-K.
Hyperkalemia treatment REVEAL-ED
This was a multicenter prospective study to determine the treatment patterns in actual practice for hyperkalemia. They found that insulin/glucose was given in 64%. Hypoglycemia occurred in 6%; 17% if K >7mmol/L. Medical therapy dropped potassium from 6.3 to 5.3 in the first 4 hours. Only hemodialysis dropped it to normal in 4 hours (dropped 2.2mmol/L). There was tremendous practice variation, with 43 different medical treatment combinations observed. Other than insulin/glucose, treatments included: “calcium (55%), inhaled b2-agonists (33%), oral sodium polystyrene sulfate (31%), IV bicarbonate (29%), dialysis (24%), and i.v. diuretics (5%).” ECG changes were only seen in about one quarter of patients. However, with a potassium >7 mmol/L, 45% of ECGs had either peaked T waves or wide QRS. The study was funded by AstraZeneca, which makes a drug to treat hyperkalemia; several authors had received funding from ZS Pharma (part of AstraZeneca) or AstraZeneca. Their drug, patiromer, is not mentioned, as it had just received FDA approval at the start of this study. However, this study provided valuable intelligence in how and where to best use their drug, which explains their interest in funding it.
Another Spoonful
Rob Orman’s ERcast has a free audio episode on hyperkalemia that is absolutely fantastic.
This emDocs Post discusses evidence-based hyperkalemia treatment.
Source
Real World Evidence for Treatment of Hyperkalemia in the Emergency Department (REVEAL-ED): A Multicenter, Prospective, Observational Study. J Emerg Med. 2018 Dec;55(6):741-750. doi: 10.1016/j.jemermed.2018.09.007. Epub 2018 Nov 1.
Open in Read by QxMD








