Authors: David Cisewski, MD (@dhcisewski, EM Resident Physician, Icahn SoM at Mount Sinai) and Courtney Cassella, MD (@Corablacas, EM Resident Physician, Icahn SoM at Mount Sinai) // Edited by: Alex Koyfman, MD (@EMHighAK, EM Attending Physician, UT Southwestern Medical Center / Parkland Memorial Hospital) and Brit Long, MD (@long_brit)
Case
A 48-year-female is brought in by EMS after being found agitated and argumentative with a parking attendant who she indicates “was trying to steal my identity” while leaving a parking garage. EMS indicates she refused finger stick on way to ED and had one episode of nausea and vomiting prior to arrival. The patient appears restless and anxious as she paces back and forth in triage, yelling with rapid speech at EMS for bringing her to the ED. She appears thin, athletic, and well-dressed, with good hygiene and no direct evidence of alcohol or drug intoxication. The patient denies history of drug or alcohol use and denies medical or surgical history, though she is tangential. She is complaining repeatedly, “everyone wants to put me jail because I’m too good at my job”. These is no witnesses or family present to corroborate her medical and social history.
VS – HR 138, BP 168/105, Temp 100.1F, RR 22 (99% on RA), FS 86
Exam: CN2-12 intact, AOX3, skin warm to touch, lungs CTABL, abdomen diffusely tender… nodular goiter felt along thyroid
Final Dx – Thyroid Storm
Psychosis, by definition, is a ‘fundamental derangement of the mind characterized by defective or lost contact with reality’ (DSM-V, Wakefield). Included in this definition is a distorted sense of reality characterized by delusions, hallucinations, and disorganized thought or behavior. These are a non-specific constellation of signs and symptoms that impair one’s ability to understand reality that are often dismissed as psychiatric in nature. It’s important to note that psychosis is not always a psychiatric illness per se, but often an indication of an organic illness. Many who have read Susannah Cahalan’s riveting novel, Brain on Fire: My Month of Madness, have developed a new-found apprehension about misdiagnosing psychosis as a symptom of underlying mental disorder (it was misdiagnosed in the ED!). Particularly in emergency medicine, we face the tendency to refer these altered patients to psychiatric services or battle the subconscious assumption that many of these patients created their symptoms (drug abuse, alcoholism).
However, as the adage goes “we cannot make the diagnosis if it’s not on our differential”. We must remember there are a number of psychosis mimics that should always be reviewed prior to making a premature diagnosis. Before reviewing the list of psychosis mimics we should review our approach to all patients presenting with psychosis. 13-23% of people experience psychotic symptoms at some point in their lifetime resulting from a number of organic causes (Perala, 2007; van Os, 2001). The presence of the following are more consistent with an organic cause of psychosis (Sheitman, 1997):
- Acute onset
- >40 yo w/o previous history of psychiatric diagnosis
- Presents in general medical or intensive care settings
- Non-auditory hallucinations (eg, visual, tactile, olfactory)
- Abnormal vital signs
- Lethargy
- Clouded sensorium
- Disorientation
- Memory loss
- Acute change from baseline function
- Coexisting medical conditions
- ***timing is sudden and reaches maximum severity acutely
The following 4 factors that favor organic vs. functional psychosis can be used as triage method (Dubin, 1983):
- Abnormal vital signs (T > 37.7, RR 25, HR 100, BP 160)
- Clouded mental status (inability to follow train of thought)
- >40 yo w/ no psych history
- Disorientation (if miss at least two when asked: day, month, year, city, place)
The following is a review of can’t-miss organic causes that should be considered when assessing the patient with psychosis:
- Infectious – cystitis, meningitis, encephalitis, herpes encephalitis, shock (septic)
- Neuro – stroke, tumor, seizure
- Pulmonary (hypoxia) – pneumonia, asthma attack, COPD exacerbation
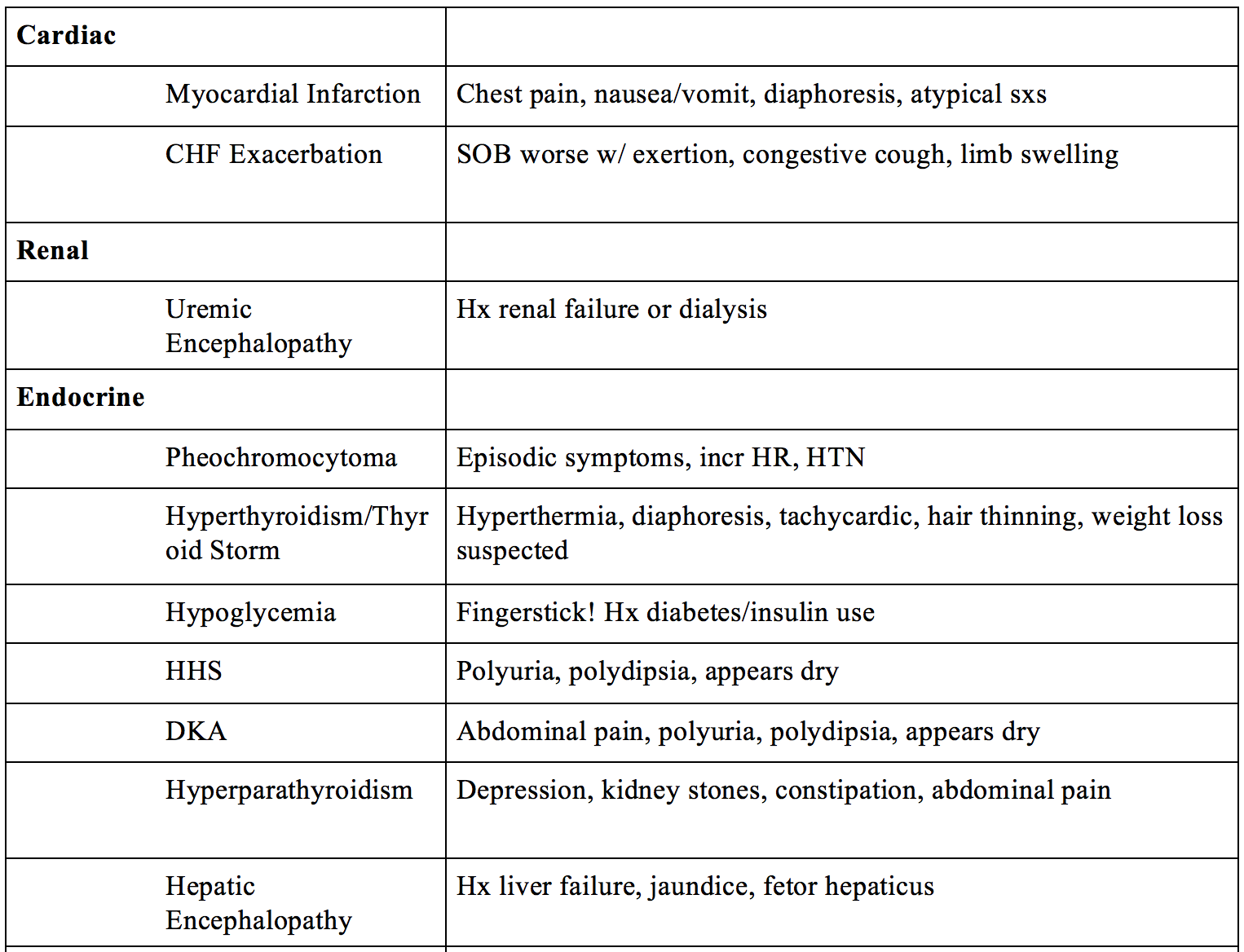
- Cardiac – myocardial infarction, CHF exacerbation
- Renal – uremic encephalopathy (ESRD)
- Metabolic/Endocrine – pheochromocytoma, hyperthyroidism, thyroid storm, hypoglycemia, significant hyperglycemia (HHS), hyperparathyroidism, DKA, hepatic encephalopathy
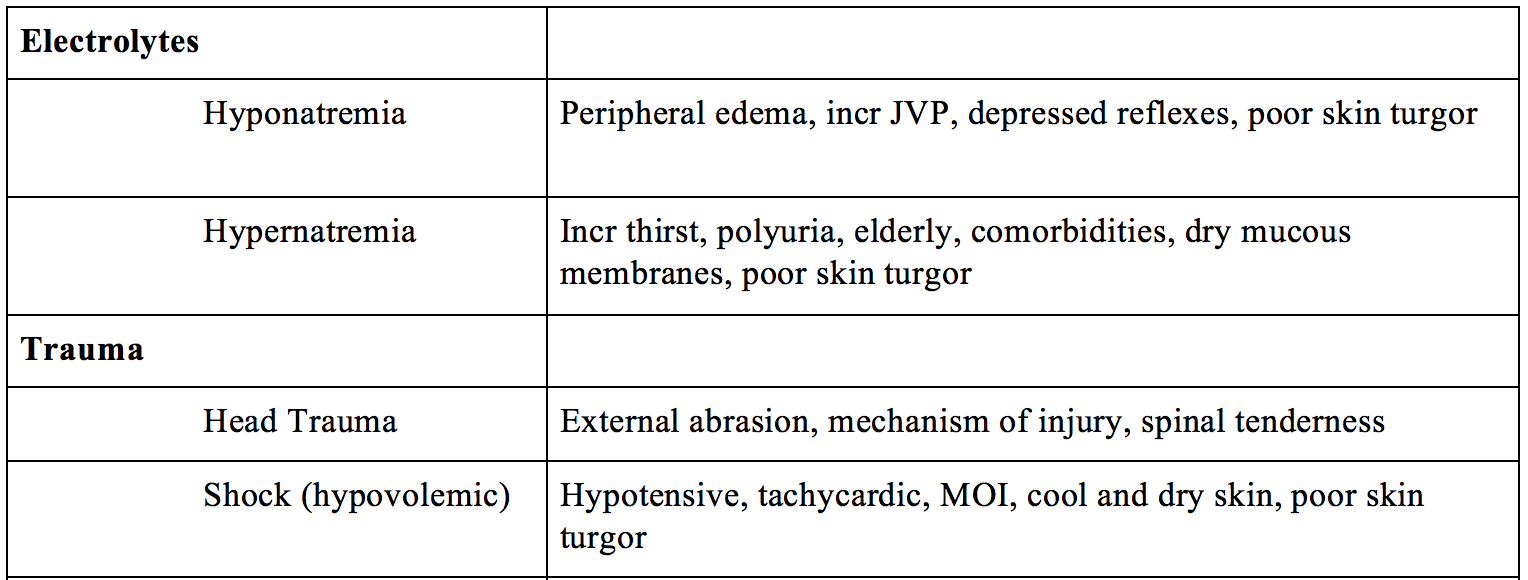
- Electrolytes – hypo/hypernatremia, hypo/hypercalcemia, hypo/hyperkalemia
- Trauma – head trauma, shock (hypovolemic)
- Toxins/Drugs – polypharmacy, steroid psychosis, anticholinergics
- Alcohol – Alcohol withdrawal or Korsakoff Psychosis
- Substance abuse – Cocaine, MDMA (ecstasy), PCP, LSD, K2, Bath salts, alpha-PVP (Flakka, Gravel), dextromethorphan (“poor man’s PCP”), Promethazine (Lean)
Infectious
Any elderly patients with a fever and/or acute change in mental status – confusion, delirium, altered mental status, agitation – particularly women from a healthcare facility or prolonged hospital stay, must be suspected of a urinary tract infection (Mody, 2014). Classic symptoms such as dysuria and suprapubic pain may be vague and non-discernible in the elderly, prompting a further discerning eye on other presenting symptoms. Early assessment of UA and UC is high on the workup list.
Classic signs of fever, nuchal rigidity, altered mental status (confusion, agitation, hallucinations), headache, nausea, and vomiting can be seen in meningitis and encephalitis. Note that the classic triad of fever, AMS, neck stiffness is present in 44% of patients (van de Beek, 2004). Clinical clues include photosensitivity, rash (meningococcal), herpetic lesions, or viral exanthema. Physical exam should look for Brudzinski Sign (spontaneous flexion of hip with passive flexion of head) or Kernig Sign (resistance to leg extension with hips flexed to 90 degrees); however, the sensitivity of these signs is poor (Thomas, 2002). The diagnosis is ultimately made with an LP +/- CT (CT should be obtained for papilledema, focal neurologic deficits, new onset seizure, GCS <10) (Brouwer, 2012).
After receiving increased media attention due to the publication of Susannah Cahalan’s, Brain on Fire, Anti-NMDA-R Encephalitis should also be considered in acute psychosis (note – it was missed twice in her two separate ED visits!). Though the incidence remains unknown, studies suggest anti-NMDA-R encephalitis is more common than HSV-1, WNV, or VZV encephalitis (Gable, 2012). Interestingly, it is suspected of being an underlying cause of historical accounts of demonic possession. Though once assumed to be a paraneoplastic syndrome secondary to ovarian teratomas, it has since been demonstrated in both males and females with an average age of 21 years old (Irani, 2008). Symptoms present over four stages (Dalmau, 2008). Stage one shows early headaches, fever, and URI symptoms. Within two weeks, patients move into stage two (psychotic stage) resulting in memory changes, paranoia, delusions, and seizures. Stage three (catatonic stage) involves catatonia, choreiform movement, and lip smacking. Stage four (hyperkinetic stage) occurs with autonomic instability, movement disorders, and death if untreated. Diagnosis involves an LP, which will show 90% lymphocytic pleocytosis, +/- oligoclonal bands (like MS). Anti-NMDA-R antibodies must be tested for in CSF. Treatment involves IVIG and aggressive steroids.
Sepsis should also be considered in acute psychosis, and early vital sign assessment is essential. Sepsis must always be considered in a patient who meets 2 or more SIRS criteria (Levy, 2003):
- T >38 or <36C
- RR >20 or PaCO2 <32mmHg
- HR >90
- WBC >12k or <4k or >10% bands
For a more detailed discussion and review on sepsis in the ED, please review a prior emDOCS article: Ready for the New Sepsis 3.0?
Neurologic
Most EM physicians will quickly recognize the classic triad of facial paresis, arm drift, and slurred speech which make up the Cincinnati Stroke Scale. (Kothari, 1997). However, what happens when the initial presentation is slurred speech alone and the patient becomes agitated and confused when the physician is not understanding them or they can’t use the words they wish to use? This could be an easy miss in an ED full of similarly presenting intoxicated or delirious patients. Conducting a focused neurological exam (complemented by a HiNTS exam if acute vestibular syndrome is present) for full evaluation of these patients can make this diagnosis. Early diagnosis with CT is required, as well as treatment with either neurosurgery or thrombolytics for a potentially time-sensitive condition.
Brain tumors can also cause altered mental status. The current incidence of brain tumor in the U.S. population is approximately 29/100,000. With headache (40-80% tension-type) being the most common initial manifestation, one would not be surprised that the ED will be the first to see many of these patients (Valentinis, 2011). In addition, they may present with nausea/vomiting, altered mental status, seizures, and other focal neurological deficits depending on the area of the brain affected. The approach to imaging in making this diagnosis should be based on focused neurologic exam and constellation of presenting symptoms. A head CT is often warranted, but when clinical suspicion for brain tumor remains high, an MRI w/ contrast is the gold standard (Chen, 2008).
Clinical symptoms such as convulsions, muscle jerking, mouth foaming, and eye rolling resulting in drowsiness, enuresis, or lateral tongue laceration suggest seizure. However, mild symptomatology and a patient’s inability to answer questions can be mistaken for psychosis. Clinical markers such as elevated lactate are useful, but when combined with elevated serum prolactin concentrations, are more useful in identifying epileptic vs. psychogenic seizure (Matz, 2016). However, these should only function as adjuncts to clinical history and exam.
Pulmonary
Pneumonia, COPD, and acute asthma exacerbations can manifest with symptoms similar to anxiety and delirium. Patients presenting with dyspnea should receive prompt administration of oxygen and an immediate evaluation for root cause of the respiratory distress. The use of pulse oximetry and VBG can be used for rapid assessment of respiratory compensation, particularly when peak expiratory flow (PEF) is unavailable in agitated patients (Hodder, 2012). US evaluation of the pulmonary system for B lines, pneumothorax, and effusion can assist providers.
Cardiac
Severe hemodynamic stability secondary to ACS/MI and CHF exacerbation can present with confusion, delirium and agitation in association with the pain and physiologic stress of the event. Elderly or chronically ill patients with less capacity for cardiovascular compensation may also progress more quickly toward a state of instability prompting the need for rapid evaluation and identification of the underlying cause (Alexander, 2007). An ECG complemented by lab troponins, BMP, and BNP are fundamental in the assessment. Ultrasound examination of cardiac activity and lungs fields should be included.
Renal
ESRD can result in the accumulation of toxins and loss of internal homeostasis leading to uremic encephalopathy (Tintinalli, 2015). This becomes fatal without renal transplant or hemodialysis. Be cautious of any patient with missed/skipped dialysis appointments. Recall that uremia is a clinical diagnosis of uremic toxin buildup, whereas azotemia is the asymptomatic elevation of nitrogen in blood. The encephalopathy caused by this uremia results in a constellation of neurologic symptoms that typically improve with dialysis. Symptoms include lethargy, irritability, disorientation, hallucinations, rambling speech, unsteady gait and weakness, tremor, myoclonus, and asterixis (Bolton, 1990). Diagnosis is clinical, not made by elevated urea, though BUN levels greater than 60 are associated with greater risk of complications (pericardial effusion/pericarditis). Treatment involves reversal with dialysis, although a lag time of 1 to 2 days is usually required before mental status clears (Bolton, 1990, Young, 1998).
Metabolic/Endocrine
Intuitively one can imagine that a catecholamine surge secondary to agitation, physiologic stressor, delirium, or a pheochromocytoma would all present similarly. Agitation or anxiety along with the classic triad of headache, palpitation, and diaphoresis is concerning for pheochromocytoma (Baguet, 2004). Symptoms are present in approximately 50% of patients with pheochromocytoma, and when present, they are typically paroxysmal. Diagnosis involves plasma fractionated metanephrines and/or 24h urine metanephrines (Cotesta, 2005). Treatment involves alpha blockers (phenoxybenzamine), followed by beta blockers.
Thyroid storm has been described as “The Great imitator” in the ED. Symptoms such as anxiety, hyperactivity, rapid speech, emotional lability, weakness, tremor, palpitations, heat intolerance, increased perspiration, and weight loss despite a normal or increased appetite are all non-specific symptoms seen during hyperthyroidism (Trzepacz, 1989). In addition, high-output heart failure, pulmonary edema, or dyspnea on exertion can occur. Vital signs show hyperthermia, hypertension, tachycardia, and AMS (Akamizu, 2012). Unique features of hyperthyroidism include a deep stare (lid retraction) and lid lag, warm and moist skin, thin and fine hair, tremors, proximal muscle weakness, and hyperreflexia. This is 100% fatal without treatment (20-50% mortality with treatment). Diagnosis is clinical and is assisted by TSH and serum free T3/T4. Treatment involves a beta blocker such as propranolol (target HR <100), PTU/Methimazole, and glucocorticoids. Some recommend avoidance of active cooling with ice packs and cooling blankets, which will precipitate peripheral vasoconstriction and worsen the hypertension (Devereaux, 2014). Treatment of the underlying etiology is warranted (especially infection).
Symptomatic hypoglycemia is typically seen at serum glucose levels less than 55-70. Anxiety and agitation are often the presenting symptoms of hypoglycemia-induced psychosis. Additional symptoms include tremor, diaphoresis, weakness, tachycardia, nausea, or vomiting. At glucose less than 50, development of confusion, visual changes, headache, and mental slowing can occur (Cryer, 2007). It is important to identify the patient’s medical history, complete a medication reconciliation (insulin, metformin, sulfonylureas, beta blockers), and look for signs of underlying infection that may have precipitated the event (Cryer, 2009). An early fingerstick is essential in the diagnosis (serum c-peptide if suspect intentional insulin overdose). Treatment involves 1 amp D50 IV followed by D5W infusion until tolerating oral ingestion (Cryer, 2009).
Severe (symptomatic) hyperglycemia is typically seen in patients with DKA or HHS. Symptoms include polyuria, polydipsia, nausea/vomiting, but also visual changes and altered mental status if severe (Pais, 2007). Recall that HHS occurs with slower onset, whereas DKA is more acute, thus timing of symptoms is useful. It is essential to obtain a detailed medical history, focusing on any changes in medication dosage or usage and accidental or intentional overdose. History of recent illness and alcohol or drug use is also important, as these may trigger an exacerbation. Rapid glucose assessment is essential for diagnosis, and fluids are key to treatment. Insulin will also be started, but be sure to check the potassium before starting (maintain 4-5) (Walls, 2009).
Hypercalcemia presents with the classic “stones (renal stones), bones (aching bones), groans (constipation), and psychiatric overtones (depression)” (Inzucchi, 2004). Hyperparathyroidism, typically the result of a parathyroid adenoma or hyperplasia, is the most common cause of hypercalcemia (Perrier, 2005). Diagnosis is made by analyzing serum calcium, PTH, and ALP levels, all of which will be elevated. EKG typically demonstrates prolonged PR, widened QRS, and shortened QT. Treatment involves IV fluids first, then calcitonin, furosemide, and bisphosphonates – a regimen best determined by an endocrine consult!
Hepatic encephalopathy should be suspected in patients with alcoholism, presentation c/w cirrhosis, or known cirrhosis. Signs and symptoms include sleep disturbance, euphoria, asterixis, slurred speech, disorientation, slurred speech, inappropriate behavior, nystagmus, confusion, and somnolence (Khungar, 2012). Signs of liver failure include jaundice, asterixis, and fetor hepaticus (“breath of the dead”). The diagnosis is clinical (any patient with liver disease who presents with an altered mental status). Serum ammonia levels can be useful (though do not correlate with severity of encephalopathy) (Ong, 2002). Treatment involves 30-60mg PO Lactulose (OGT/NGT if PO intolerance) or polyethylene glycol, titrated to 2-4 stools/day. Rifaximin 400mg TID (decrease colonic bacteria) and low protein diet are also useful (O’veirne, 2012).
Electrolytes
Hyponatremia can result in fatigue, weakness, muscle cramps, and headache. If severe, hyponatremia can result in AMS, hallucinations, delirium, agitation, and confusion. (Adrogue, 2000). Quick diagnosis involves VBG (for serum Na). Note – don’t forget to correct for glucose! Underlying treatment depends on cause; treat the symptoms, not the lab value. You will usually restrict free water and give IV NS or LR as needed (correct < 0.5 mEq/L/h). (Yeates, 2004) Beware of symptoms of overcorrection, which can result in dysarthria, dysphagia, and paresis (central pontine myelinolysis) (Adrogue, 2000).
On the opposite spectrum, hypernatremia can present with irritability, headache, tremors, lethargy, delirium, and coma. Similarly, you need to treat the underlying disorder, replace free water deficit (1/2NS), and correct sodium at a rate < 0.5 mEq/L/h. Consider 20-40mg IV furosemide for further Na losses.
Trauma
Head trauma should be considered in any patient with AMS, headache, or slurred speech following a severe mechanism of injury. Evaluate for anisocoria, unstable gait, focal neurologic abnormalities, and Cushing’s Triad (hypertension, bradycardia, irregular respiratory rate) (Zammit, 2013). Treatment includes the ABCD’s of trauma; maintain normal vital signs and avoid the “H-bombs” – hypoxia, hypoglycemia, hypo/hyper-tension, hypo/hypercarbia. Elevate head of bed, consider hyperosmotic agent (hypertonic saline or mannitol), and obtain an early neurosurgery consult.
Shock (hypovolemic, distributive, cardiogenic, obstructive) may result in decreased blood flow to the brain resulting in confusion, delirium, agitation, and/or aggression. Bedside ultrasound can assist in diagnosis in the type of shock. There are several protocols available to evaluate the etiology of shock using US.
Toxins/Drugs
Polypharmacy becomes a growing problem as individuals age and develop multiple comorbidities. Inappropriate drug prescribing and/or administration can lead to adverse drug events (ADE’s) such as delirium, agitation, lethargy, and even coma. This should be particularly suspected in the geriatric population. One study showed 36% of participants between 62-85 years used five or more prescription medications (Qato, 2016). Further studies have estimated that approximately 20% of Medicare beneficiaries have five or more chronic conditions, further complicating the identification of the primarily etiology of psychosis (Tinetti, 2004). One should also consider the Beers List of medications (meds to be avoided in the elderly) (Counsell, 2015). The top 6 can be remembered with the mnemonic “BAT MAN” – Benzodiazepines, Anticholinergics, Tricyclic antidepressants, Muscle relaxants, Anti-epileptics, and Nitrofurantoin. Corroboration by family members, health care staff, or medical records is essential to make this diagnosis.
Steroid psychosis should be expected in any patient on glucocorticoid therapy for inflammatory, allergic, immunologic, and malignant disorders. Symptoms are similar to Cushing’s syndrome and from a neurologic perspective include dysphoria/depression, insomnia/akathisia, and mania/psychosis (Brown, 2001). Glucocorticoids have also been shown to increase the risk of suicidal behavior, which is often seen first in the ED (Fardet, 2012).
As mentioned within the polypharmacy section, anticholinergics are a particularly common cause of psychosis, especially among the elderly. (Carrière, 2009) Symptoms can be remembered with the mnemonic: “Mad as a hatter” (agitated, confused, bizarre behavior, visual hallucinations), “blind as a bat” (mydriasis), “hot as a hare” and “red as a beet” (peripheral vasodilation, hyperthermia), and “bloated as a toad” (urinary retention). If suspected, a trial dose of 1.0mg IV physostigmine, slow push over 5 min repeated with max of 2mg, can be both diagnostic and therapeutic.
Alcohol
 Alcohol withdrawal symptoms occur in patients who abruptly stop drinking following a prolonged period of consumption. Early symptoms include hand tremors, headache, nausea and vomiting, diaphoresis, tachycardia, hypertension, fever, agitation, and hyperarousal. If severe, it can result in seizures (5-15%), hallucinations, and delirium tremens (5%) (Kosten, 2003; Mayo-Smith, 2004; Stehman, 2013). There exists a spectrum of symptom presentation timing, ranging from 2-6 hours up to 2 weeks following cessation. Kindling refers to the increased severity of subsequent withdrawal episodes in patients with repeated events (ie, worse every time).
Alcohol withdrawal symptoms occur in patients who abruptly stop drinking following a prolonged period of consumption. Early symptoms include hand tremors, headache, nausea and vomiting, diaphoresis, tachycardia, hypertension, fever, agitation, and hyperarousal. If severe, it can result in seizures (5-15%), hallucinations, and delirium tremens (5%) (Kosten, 2003; Mayo-Smith, 2004; Stehman, 2013). There exists a spectrum of symptom presentation timing, ranging from 2-6 hours up to 2 weeks following cessation. Kindling refers to the increased severity of subsequent withdrawal episodes in patients with repeated events (ie, worse every time).
Alcohol withdrawal is especially concerning during prolonged ED stays (extensive workups can last hours). Diffuse, tonic-clonic seizures will often present, with 90% occurring within 48 hours (Tintinalli, 2015; Pitzelli, 2010). Alcoholic hallucinations occur 12-48 hours after last drink, predominantly auditory, and along with agitation, can last from days to weeks (Jordaan, 2014). Delirium tremens are the acute, fluctuating, disturbances of consciousness, confusion, and impairment in cognitive and perceptual function (Kosten, 2003; Mayo-Smith, 2004; Stehman, 2013). In his discussion of the dipsomaniacal behavior of alcoholics as an uncontrollable craving for alcohol resulting in hallucinations, Jack London became the first to coin the common term used today, ‘pink elephants’.
“The drinker [ ] who sees, in the extremity of his ecstasy, blue mice and pink elephants” –
–Jack London (John BarleyCorn, 1913).
Diagnosis of alcoholic hallucinosis/ delirium tremens is based on clinical suspicion and grading of severity (CIWA score), as well as evaluating for other conditions such as sepsis, trauma, or co-ingestions. Treatment involves repletion of fluids and electrolytes (‘banana bag’). If seizures develop, benzodiazepines are the gold standard with chronic alcoholics requiring higher doses (Mayo-Smith, 2004; Amato, 2010).
Substance abuse
Cocaine, MDMA (ecstasy), PCP, LSD, K2, Bath salts, alpha-PVP (aka, Flakka, Gravel), dextromethorphan (“poor man’s PCP”), and promethazine (Lean) can result in psychosis and can be seen in any environment, age range, sex, and socioeconomic status. Flakka (aka, gravel, poor-man’s cocaine) is an emerging drug that should be considered. Getting its name from Spanish slang for a beautiful woman (“la flaca”), it is a synthetic stimulant, similar to bath salts, which results is hyperstimulation, paranoia, hallucinations, and hyperthermia (Sauer, 2009). Diagnosis is clinical (or corroboration from acquaintances), and treatment is supportive.
Summary
In the absence of a previous history or psychiatric condition, a nonorganic cause of acute symptoms is a diagnosis of exclusion. In addition, one must never assume factitious disorder or malingering until other conditions are ruled out. When psychosis is prematurely assumed to be related to psychiatric condition versus organic cause, delay in failure to treat can be deadly. Hence, the importance of a proper review. Look for signs of organic causes, and be sure to gather a proper history. Most importantly, beware of your cognitive bias during certain patient presentations, and ensure you’re covering all possibilities before making a final diagnosis.


References / Further Reading:
Adrogue, H. J., & Madias, N. E. (2000). Hyponatremia. N Engl J Med, 342(21), 1581-1589. doi:10.1056/NEJM200005253422107
Adrogue, H. J., & Madias, N. E. (2000). Hypernatremia. N Engl J Med, 342(20), 1493-1499. doi:10.1056/NEJM200005183422006
Akamizu, T., Satoh, T., Isozaki, O., Suzuki, A., Wakino, S., Iburi, T., Japan Thyroid, A. (2012). Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid, 22(7), 661-679. doi:10.1089/thy.2011.0334
Alexander, K. P., et al. (2007). “Acute coronary care in the elderly, part I: Non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology.” Circulation 115(19): 2549-2569.
Amato, L., Minozzi, S., Vecchi, S., & Davoli, M. (2010). Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev(3), CD005063. doi:10.1002/14651858.CD005063.pub3
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association, 2013.
Baguet, J. P., Hammer, L., Mazzuco, T. L., Chabre, O., Mallion, J. M., Sturm, N., & Chaffanjon, P. (2004). Circumstances of discovery of phaeochromocytoma: a retrospective study of 41 consecutive patients. Eur J Endocrinol, 150(5), 681-686. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15132724
Bolton, CF, Young, GB. Uremic encephalopathy. In: Bolton, CF, Young, GB, (Eds), Neurological Complications of Renal Disease, Buttersworth, Stoneham 1990. P.44.
Brouwer, M. C., et al. (2012). “Dilemmas in the diagnosis of acute community-acquired bacterial meningitis.” Lancet 380(9854): 1684-1692.
Brown, E. S., & Chandler, P. A. (2001). Mood and Cognitive Changes During Systemic Corticosteroid Therapy. Prim Care Companion J Clin Psychiatry, 3(1), 17-21. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15014624
Carriere, I., Fourrier-Reglat, A., Dartigues, J. F., Rouaud, O., Pasquier, F., Ritchie, K., & Ancelin, M. L. (2009). Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med, 169(14), 1317-1324. doi:10.1001/archinternmed.2009.229
Chen, W. and D. H. Silverman (2008). “Advances in evaluation of primary brain tumors.” Semin Nucl Med 38(4): 240-250.
Cotesta, D., Caliumi, C., Alo, P., Petramala, L., Reale, M. G., Masciangelo, R., . . . Letizia, C. (2005). High plasma levels of human chromogranin A and adrenomedullin in patients with pheochromocytoma. Tumori, 91(1), 53-58. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15850005
Counsell, S. R. (2015). “2015 updated AGS Beers Criteria offer guide for safer medication use among older adults.” Geriatr Nurs 36(6): 488-489.
Cryer, P. E. (2007). Hypoglycemia, functional brain failure, and brain death. J Clin Invest, 117(4), 868-870. doi:10.1172/JCI31669
Cryer, P. E., Axelrod, L., Grossman, A. B., Heller, S. R., Montori, V. M., Seaquist, E. R., . . . Endocrine, S. (2009). Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab, 94(3), 709-728. doi:10.1210/jc.2008-1410
Dalmau, J., Gleichman, A. J., Hughes, E. G., Rossi, J. E., Peng, X., Lai, M., . . . Lynch, D. R. (2008). Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol, 7(12), 1091-1098. doi:10.1016/S1474-4422(08)70224-2
Devereaux, D., & Tewelde, S. Z. (2014). Hyperthyroidism and thyrotoxicosis. Emerg Med Clin North Am, 32(2), 277-292. doi:10.1016/j.emc.2013.12.001
Dubin, W. R., Weiss, K. J., & Zeccardi, J. A. (1983). Organic brain syndrome. The psychiatric imposter. JAMA, 249(1), 60-62. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6848782
Fardet, L., Petersen, I., & Nazareth, I. (2012). Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry, 169(5), 491-497. doi:10.1176/appi.ajp.2011.11071009
Gable, M. S., et al. (2012). “The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project.” Clin Infect Dis 54(7): 899-904.
Hodder, R., et al. (2010). “Management of acute asthma in adults in the emergency department: assisted ventilation.” CMAJ 182(3): 265-272.
Irani, S. R., Bera, K., Waters, P., Zuliani, L., Maxwell, S., Zandi, M. S., . . . Vincent, A. (2010). N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain, 133(Pt 6), 1655-1667. doi:10.1093/brain/awq113
Inzucchi, S. E. (2004). “Understanding hypercalcemia. Its metabolic basis, signs, and symptoms.” Postgrad Med 115(4): 69-70, 73-66.
Jordaan, G. P., & Emsley, R. (2014). Alcohol-induced psychotic disorder: a review. Metab Brain Dis, 29(2), 231-243. doi:10.1007/s11011-013-9457-4
Judith E. Tintinalli, e. a. (2015). Tintinalli’s Emergency Medicine: McGraw Hill.
Khungar, V., & Poordad, F. (2012). Hepatic encephalopathy. Clin Liver Dis, 16(2), 301-320. doi:10.1016/j.cld.2012.03.009
Khungar, V., & Poordad, F. (2012). Management of overt hepatic encephalopathy. Clin Liver Dis, 16(1), 73-89. doi:10.1016/j.cld.2011.12.007
Koranyi, E. K. (1979). Morbidity and rate of undiagnosed physical illnesses in a psychiatric clinic population. Arch Gen Psychiatry, 36(4), 414-419. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/426608
Kosten, T. R., & O’Connor, P. G. (2003). Management of drug and alcohol withdrawal. N Engl J Med, 348(18), 1786-1795. doi:10.1056/NEJMra020617
Kothari, R., Hall, K., Brott, T., & Broderick, J. (1997). Early stroke recognition: developing an out-of-hospital NIH Stroke Scale. Acad Emerg Med, 4(10), 986-990. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9332632
Levy, M. M., et al. (2003). “2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.” Crit Care Med 31(4): 1250-1256.
Lipowski, Z. J. (1967). Delirium, clouding of consciousness and confusion. J Nerv Ment Dis, 145(3), 227-255. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4863989
Maloney, P. J. (2013). Sepsis and septic shock. Emerg Med Clin North Am, 31(3), 583-600. doi:10.1016/j.emc.2013.04.006
Martin, G. S., Mannino, D. M., Eaton, S., & Moss, M. (2003). The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med, 348(16), 1546-1554. doi:10.1056/NEJMoa022139
Matz, O., Zdebik, C., Zechbauer, S., Bundgens, L., Litmathe, J., Willmes, K., . . . Dafotakis, M. (2016). Lactate as a diagnostic marker in transient loss of consciousness. Seizure, 40, 71-75. doi:10.1016/j.seizure.2016.06.014
Mayo-Smith, M. F., Beecher, L. H., Fischer, T. L., Gorelick, D. A., Guillaume, J. L., Hill, A., . . . Working Group on the Management of Alcohol Withdrawal Delirium, P. G. C. A. S. o. A. M. (2004). Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med, 164(13), 1405-1412. doi:10.1001/archinte.164.13.1405
Mody, L., & Juthani-Mehta, M. (2014). Urinary tract infections in older women: a clinical review. JAMA, 311(8), 844-854. doi:10.1001/jama.2014.303
O’Beirne, J. P., Chouhan, M., & Hughes, R. D. (2006). The role of infection and inflammation in the pathogenesis of hepatic encephalopathy and cerebral edema in acute liver failure. Nat Clin Pract Gastroenterol Hepatol, 3(3), 118-119. doi:10.1038/ncpgasthep0417
Ong, J. P., Aggarwal, A., Krieger, D., Easley, K. A., Karafa, M. T., Van Lente, F., . . . Mullen, K. D. (2003). Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med, 114(3), 188-193. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12637132
Pais, I., Hallschmid, M., Jauch-Chara, K., Schmid, S. M., Oltmanns, K. M., Peters, A., Schultes, B. (2007). Mood and cognitive functions during acute euglycaemia and mild hyperglycaemia in type 2 diabetic patients. Exp Clin Endocrinol Diabetes, 115(1), 42-46. doi:10.1055/s-2007-957348
Perala, J., Suvisaari, J., Saarni, S. I., Kuoppasalmi, K., Isometsa, E., Pirkola, S., . . . Lonnqvist, J. (2007). Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry, 64(1), 19-28. doi:10.1001/archpsyc.64.1.19
Perrier, N. D. (2005). “Asymptomatic hyperparathyroidism: a medical misnomer?” Surgery 137(2): 127-131.
Pitzele, H. Z., & Tolia, V. M. (2010). Twenty per hour: altered mental state due to ethanol abuse and withdrawal. Emerg Med Clin North Am, 28(3), 683-705. doi:10.1016/j.emc.2010.03.006
Qato, D. M., Wilder, J., Schumm, L. P., Gillet, V., & Alexander, G. C. (2016). Changes in Prescription and Over-the-Counter Medication and Dietary Supplement Use Among Older Adults in the United States, 2005 vs 2011. JAMA Intern Med, 176(4), 473-482. doi:10.1001/jamainternmed.2015.8581
Sauer, C., Peters, F. T., Haas, C., Meyer, M. R., Fritschi, G., & Maurer, H. H. (2009). New designer drug alpha-pyrrolidinovalerophenone (PVP): studies on its metabolism and toxicological detection in rat urine using gas chromatographic/mass spectrometric techniques. J Mass Spectrom, 44(6), 952-964. doi:10.1002/jms.1571
Sheitman, B. B., Lee, H., Strous, R., & Lieberman, J. A. (1997). The evaluation and treatment of first-episode psychosis. Schizophr Bull, 23(4), 653-661. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9366001
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., . . . Angus, D. C. (2016). The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA, 315(8), 801-810. doi:10.1001/jama.2016.0287
Stehman, C. R., & Mycyk, M. B. (2013). A rational approach to the treatment of alcohol withdrawal in the ED. Am J Emerg Med, 31(4), 734-742. doi:10.1016/j.ajem.2012.12.029
Tinetti, M. E., Bogardus, S. T., Jr., & Agostini, J. V. (2004). Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med, 351(27), 2870-2874. doi:10.1056/NEJMsb042458
Thomas, K. E., et al. (2002). “The diagnostic accuracy of Kernig’s sign, Brudzinski’s sign, and nuchal rigidity in adults with suspected meningitis.” Clin Infect Dis 35(1): 46-52.
Trzepacz, P. T., Klein, I., Roberts, M., Greenhouse, J., & Levey, G. S. (1989). Graves’ disease: an analysis of thyroid hormone levels and hyperthyroid signs and symptoms. Am J Med, 87(5), 558-561. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2816972
Valentinis, L., Tuniz, F., Valent, F., Mucchiut, M., Little, D., Skrap, M., . . . Zanchin, G. (2010). Headache attributed to intracranial tumours: a prospective cohort study. Cephalalgia, 30(4), 389-398. doi:10.1111/j.1468-2982.2009.01970.x
van de Beek, D., et al. (2004). “Clinical features and prognostic factors in adults with bacterial meningitis.” N Engl J Med 351(18): 1849-1859.
van Os, J., Hanssen, M., Bijl, R. V., & Vollebergh, W. (2001). Prevalence of psychotic disorder and community level of psychotic symptoms: an urban-rural comparison. Arch Gen Psychiatry, 58(7), 663-668. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11448373
Victor, M., & Brausch, C. (1967). The role of abstinence in the genesis of alcoholic epilepsy. Epilepsia, 8(1), 1-20. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4961509
Wakefield, Jerome C. DSM-5: An Overview of Changes and Controversies. Clinical Social Work Journal. 2013:41 (2): 139–154.
Walls, Ron; John J. Ratey; Robert I. Simon (2009). Rosen’s Emergency Medicine: Expert Consult Premium Edition – Enhanced Online Features and Print (Rosen’s Emergency Medicine: Concepts & Clinical Practice (2v.)). St. Louis: Mosby.
Yeates, K. E., Singer, M., & Morton, A. R. (2004). Salt and water: a simple approach to hyponatremia. CMAJ, 170(3), 365-369. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14757675
Young, GB, DeRubeis, DA. Metabolic encephalopathies. In: Young, GB, Ropper, AH, Bolton, CF, (Eds), Coma and Impaired Consciousness, McGraw-Hill, 1998. p.307.
Zammit, C., & Knight, W. A. (2013). Severe traumatic brain injury in adults. Emerg Med Pract, 15(3), 1-28. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23452439









1 thought on “Psychosis Mimics: ED Differential Diagnosis and Keys to Management”
Pingback: Länkar v43- | Internmedicin