Authors: Adrianna Long, MD (EM Senior Resident at SAUSHEC, USA) and Brit Long, MD (@long_brit – EM Chief Resident at SAUSHEC, USAF) // Edited by: Alex Koyfman, MD (@EMHighAK – EM Attending Physician, UTSW / Parkland Memorial Hospital) and Manpreet Singh, MD (@MPrizzleER – Clinical Instructor & Ultrasound/Med-Ed Fellow / Harbor-UCLA Medical Center)
Sepsis can be life-threatening and is a commonly managed condition in the emergency department. This area is heavily researched, with studies evaluating multiple aspects of sepsis including evaluation, management, antibiotic use, intravenous and vasopressor resuscitation, and monitoring. One specific area of research has focused on fluid resuscitation in sepsis, specifically the type and amount of intravenous fluid. With all of the new sepsis updates, what is the literature on the harms of over-resuscitation?
The evidence continues to indicate that over-aggressive fluid resuscitation in septic shock is associated with increased morbidity and mortality. While the Sepsis Campaign Guidelines indicate that a patient with sepsis and hypotension or an elevated lactate (≥4mmol/L) should be treated with a 30ml/kg dose of crystalloid fluids, there is a lack of evidence to support this recommended fluid dose.1
In previous discussions, we have addressed that IV fluid choices affect patient outcomes in septic shock, and we have shown the evidence that invasive monitoring coupled with aggressive treatments are actually harming our patients. Please refer to these prior posts for more information:
The question we now face is what is the result of over-resuscitation?
The results of the ProCESS, ARISE, PROMISE trials indicate that EGDT as defined by Rivers et al may be more invasive than what is actually necessary to provide adequate resuscitation for patients in septic shock.2-5 These trials did not address the question of potential harm with excessive fluid administration to our patients during resuscitation. The majority of the patients in these trials received approximately 1.5L to 3L of fluids in the first 6 hours of treatment, with resuscitation approaching 4L after 6 hours.
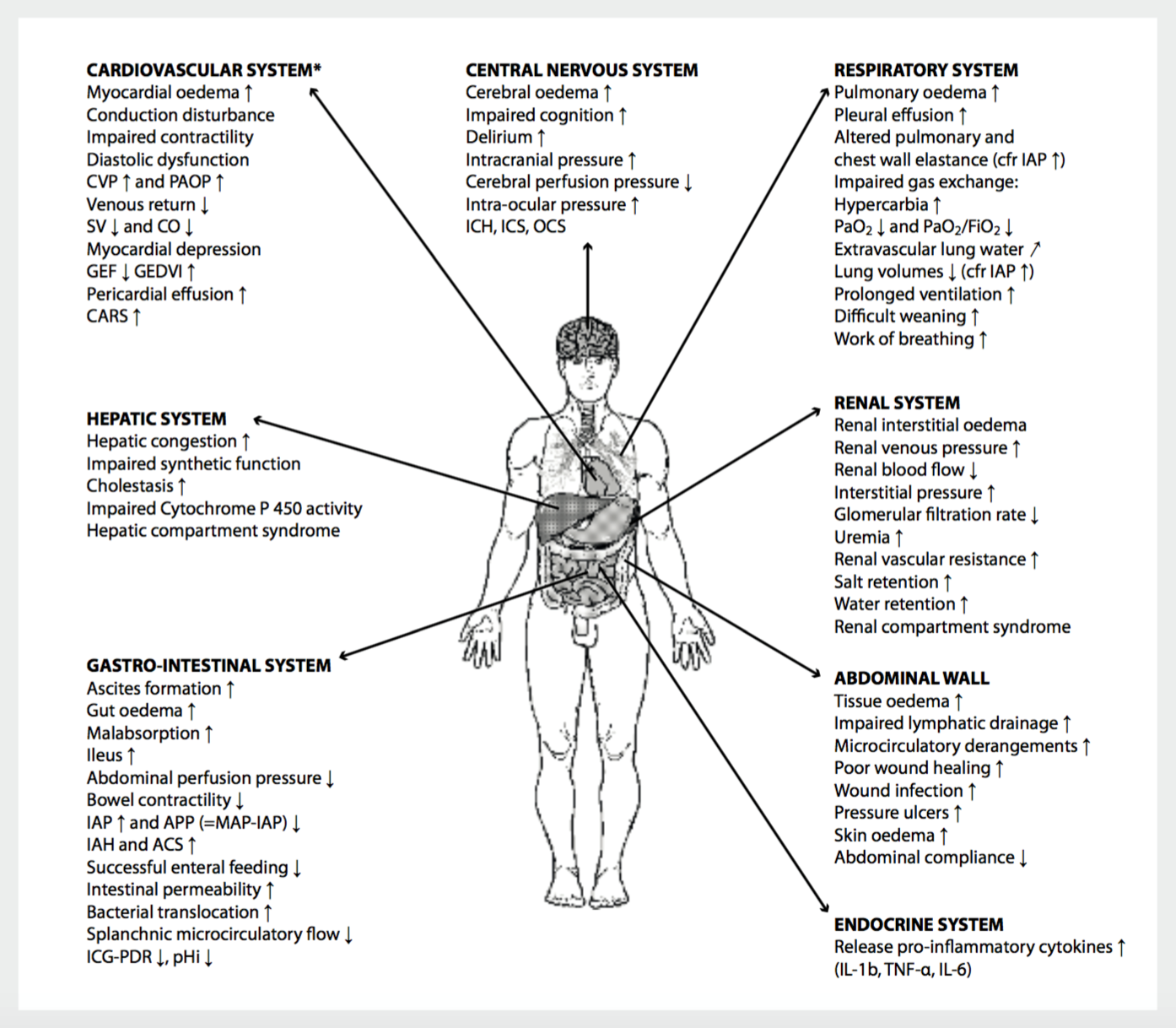
Evidence is now indicating that intravenous fluids may be harmful in patients with septic shock, as excess fluid may cause edema in the lungs, kidneys, and brain amongst other organ systems.6 All organ systems are affected with resuscitation and excess fluid, which is shown in Figure 1.
Figure 1 – Over-resuscitation effects on organ systems from Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van Regenmortel N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014 Nov-Dec;46(5):361-80.
A more clearly defined endpoint of resuscitation in goal directed therapy should be defined in order to prevent fluid overload.7 To date, finding this endpoint has been problematic. Multiple studies have shown that a positive fluid balance is associated with increased mortality.8-12 One study found that a negative fluid balance in patients with septic shock was associated with increased survival.13 This study consisting of 36 patients admitted to the ICU found improved outcomes in patients with a negative fluid balance in the first 3 days of admission.
The FEAST trial explored the effects of fluid treatment in septic children and showed an increased 48-hour mortality in children who received more fluids, specifically bolus fluids. This was a randomized controlled trial conducted across several sites in Africa. The patients were ages 60 days to 12 years with severe infection, fever, and impaired perfusion. Patients were randomized to treatment arms of albumin bolus (20-40ml/kg), saline bolus (20-40ml/kg), or no fluid bolus.9 Patients receiving no bolus demonstrated a 3.3% survival benefit at 48 hours over the groups receiving bolus fluids. Of note, this study contained a high percentage of patients with malaria, anemia (1/3 of patients had a hemoglobin < 5 g/dL) and respiratory distress (80% of patients). Few patients were included with severe hypotension, and these patients were given bolus fluids. The mechanism of excess mortality has been attributed to refractory septic shock or cardiogenic shock in patients treated with the higher doses of fluids.14,15
The SOAP study was an observational study of adults with sepsis that showed an association between a higher cumulative fluid balance in the first 72-hours of onset of sepsis and increased mortality.11 This study of 3,147 patients found predictors of poor prognosis included age, septic shock, cancer, and positive fluid balance.
One prospective observational study of patients with septic shock questioned whether the amount of initial IV fluids and cumulative IV fluids over the initial 72-hour period was associated with a higher mortality. This study included 364 patients and showed that initial fluid volume and total cumulative 72-hour fluid volume were not associated with increased mortality, which at first glance contrasts with the evidence published from the other studies listed above.16 At three days, patients with continued shock receiving more fluids demonstrated lower mortality. However, at 72 hours, patients on average had received 7.5L, vastly decreased from other studies approaching 20L. These other studies demonstrated worse outcome with this fluid amount compared to patients receiving less.
This illustrates the need for randomized control trials to identify the appropriate amount of fluid that should be administered to patients with septic shock. These prior trials included patients of different ages, comorbidities, illness severity, and most importantly, differing definitions of the amount of fluid.
What does excess fluid do?
Several explanations exist for why excess fluid causes harm. These include release of natriuretic peptides in the setting of hypervolemia resulting in vasodilation, disruption of the glycocalyx, loss of physiologic compensation (sympathetically mediated) leading to cardiovascular collapse, fluid overload resulting in cardiotoxicity, increased interstitial edema, impaired gas exchange, and acid-base and electrolyte disturbances.17-19 As detailed in Figure 1, all organ systems may be affected by excess fluid.
Wait, the glycocalyx?
The glycocalyx is a thin layer containing several types of protein. The components include proteoglycans, glycoproteins, albumin, and glycosaminoglycans which form a tight network of negatively charged ions. This layer is thought to maintain vascular permeability, mediate nitric oxide production, retain vascular protective enzymes, and modulate inflammatory markers such as cytokines. Disruption of this layer may further edema, inflammation, hypercoagulability, platelet aggregation, and sepsis syndromes including capillary leak. Studies are underway evaluating risk factors contributing to glycocalyx damage, other mechanisms of damage, and treatments aimed towards the glycocalyx.20-22
So what should the emergency provider do?
First, recognize that resuscitation goals include obtaining adequate perfusion pressure and microcirculatory flow, while limiting extra tissue edema. These measures can be completed using adequate fluid loading, as the patient still requires fluids for preload. Providing three to four liters of crystalloid will likely not harm the patient, but will improve perfusion pressure and microcirculatory flow. Second, infusing peripheral vasopressors, specifically norepinephrine, for patients with poor perfusion following this fluid load is recommended to provide peripheral squeeze, further increasing preload.
Measure the response through multiple measures, rather than relying on just one. Closely evaluate urine output, capillary refill, mental status, and IVC variation on bedside US in combination. Unfortunately microcirculatory endpoints are currently not feasible in the ED, but many are undergoing validation for use in the critical care setting.
Summary:
The dosing of intravenous fluids in septic patients should be taken as seriously as any potentially lethal medication. It is essential for physicians to give appropriate doses of intravenous fluids while avoiding fluid overload. Patients’ fluid status must be re-evaluated after administration of fluids. Further research must be conducted to identify the appropriate dosing of intravenous fluid bolus at onset of sepsis and any patient subsets that require different treatment.
References/Further Reading
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580-637.
- Pro CI, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693.
- Investigators A, Group ACT, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-1506.
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301-1311.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
- Durairaj L, Schmidt GA. Fluid therapy in resuscitated sepsis: less is more. Chest. 2008;133(1):252-263.
- Kozek-Langenecker SA. Intravenous fluids: should we go with the flow? Crit Care. 2015;19 Suppl 3:S2.
- Sadaka F, Juarez M, Naydenov S, O’Brien J. Fluid resuscitation in septic shock: the effect of increasing fluid balance on mortality. J Intensive Care Med. 2014;29(4):213-217.
- Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483-2495.
- Payen D, de Pont AC, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74.
- Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344-353.
- Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259-265.
- Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest. 2000;117(6):1749-1754.
- Maitland K, George EC, Evans JA, et al. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68.
- Myburgh J, Finfer S. Causes of death after fluid bolus resuscitation: new insights from FEAST. BMC Med. 2013;11:67.
- Smith SH, Perner A. Higher vs. lower fluid volume for septic shock: clinical characteristics and outcome in unselected patients in a prospective, multicenter cohort. Crit Care. 2012;16(3):R76.
- Glassford NJ, Eastwood GM, Bellomo R. Physiological changes after fluid bolus therapy in sepsis: a systematic review of contemporary data. Critical care. 18(6):696. 2014.
- Hilton AK, Bellomo R. A critique of fluid bolus resuscitation in severe sepsis. Crit Care. 2012;16:(1)302.
- Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van Regenmortel N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014 Nov-Dec;46(5):361-80.
- Chappell D, Westphal M, Jacob M. The impact of the glycocalyx on microcirculatory oxygen distribution in critical illness. Curr Opin Anaesthesiol. 2009 Apr;22(2):155-62.
- Burke-Gaffney A, Evans TW. Lest we forget the endothelial glycocalyx in sepsis. Crit Care. 2012 Dec 12;16(2):121.
- Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010 Jul 15;87(2):300-10.









2 thoughts on “The Dangers of Over-Resuscitation in Sepsis”
Pingback: Länkar v23 | Internmedicin
Pingback: Asynchronous Learning: Infectious Disease, Immunology, and Dermatology - Bold City Emergency Medicine