Author: John Cooper, DO (EM Resident Physician, UT Southwestern Emergency Medicine Residency) // Edited by: Alex Koyfman, MD (@EMHighAK) and Brit Long, MD (@long_brit)
Introduction
It’s the dead cold of winter, and your ED has turned into a dreary wasteland of coughing patients. You pick up a chart and go see your next patient: a 17-year-old male you had seen one week before for fever and congestion, diagnosed with a viral upper respiratory infection. He has a history of intellectual disability from a stroke suffered as a neonate and subsequently is not the best historian. He returns this time for nasal congestion with headache. He endorses a severe frontal headache, fevers, sore throat, and nasal drainage. When he looks around the room, he gets dizzy and is seeing double. He is febrile, tachycardic, and ill appearing, and you note proptosis of the right eye, a sluggish pupil, and inability to move the eye due to pain. Also noted is facial tenderness over the right maxillary sinus and right nasal congestion with purulent drainage and post nasal drip. How do we differentiate this patient whose initial presentation was a simple upper respiratory infection? We will discuss this as we talk about sinusitis and its relationship to several other potentially dangerous conditions.
Background
Sinusitis is common, very common. As many as 31 million Americans will experience at least one episode annually. [1] Given this is such a common diagnosis, emergency clinicians must consider other conditions, other than just the run-of-the-mill stuffy nose. So how do sinusitis mimics present? How are they different, and what are their similarities?
Overall, you can classify sinusitis by time and symptoms. Acute sinusitis lasts less than 4 weeks, subacute 4-12 weeks, chronic > 12 weeks, and recurrent with 4 or more episodes in a 12-month period. Sinusitis classification is based on the clinical picture of viral, uncomplicated bacterial, or complicated bacterial. [2]
The American Academy of Otolaryngology-Head and Neck Surgery’s most recent 2015 guidelines for the diagnosis of acute bacterial sinusitis are overall simple. Signs and symptoms of acute rhinosinusitis include purulent nasal drainage, nasal obstruction, facial pain, facial pressure persisting greater than 10 days without improvement, or symptoms worsening within 10 days after initially improving. [2] The diagnosis of acute bacterial sinusitis in children is slightly different, but with similar guidelines. The AAP simplified its criteria for diagnosis of acute bacterial to persistent symptoms, cough, or nasal discharge to > 10 days, worsening of symptoms after initial improvement, or severe onset of symptoms with fever > 39 C for 3 days. [3]

Clinically, the feature of sinusitis that most commonly presents as other conditions is facial pain and/or headache. This is due to the shared innervation of the various sinuses and other facial structures that create a varied presentation. Other symptoms such as fever, nasal drainage, anosmia, and cough also are nonspecific. [4] Physical exam may include purulent drainage from the nose and pain with percussion of the sinus. However, exam findings do not possess good sensitivity or specificity. [5]
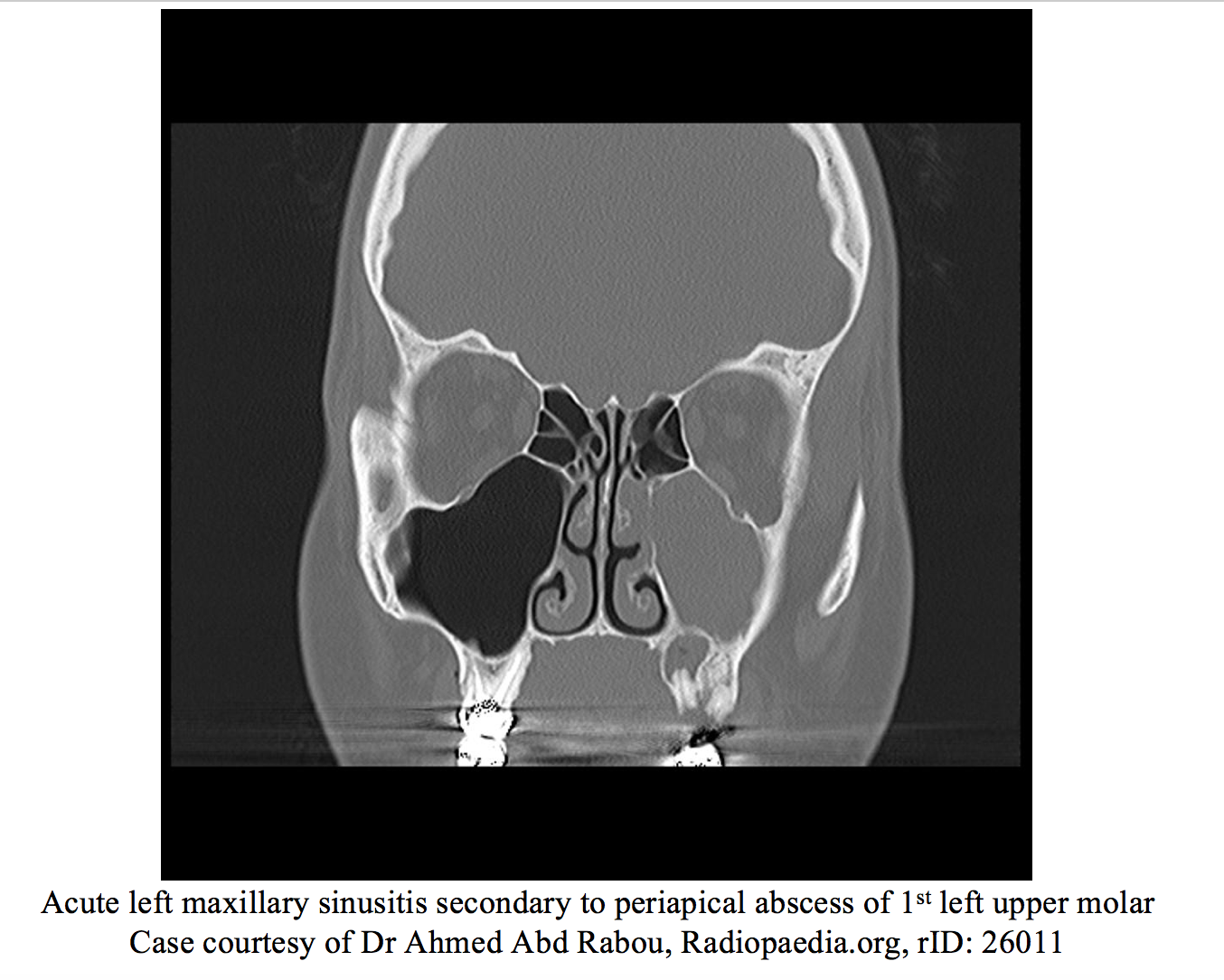
Imaging can be useful for diagnosis but is overall non-specific. In addition, it does nothing to help distinguish between bacterial and viral causes of sinusitis. CT may show mucosal thickening or air fluid levels suggestive of sinusitis, but these findings are not specific to the diagnosis. [6] Mucosal thickening can be seen in upwards of 50% of asymptomatic individuals.
Individual treatment for sinusitis is variable. Given sinusitis is usually a self-limited disease, watchful waiting with symptomatic treatment is often recommended. Antibiotics can be held and started should patients fail to improve or if they worsen. [2]
Cavernous Sinus Thrombosis
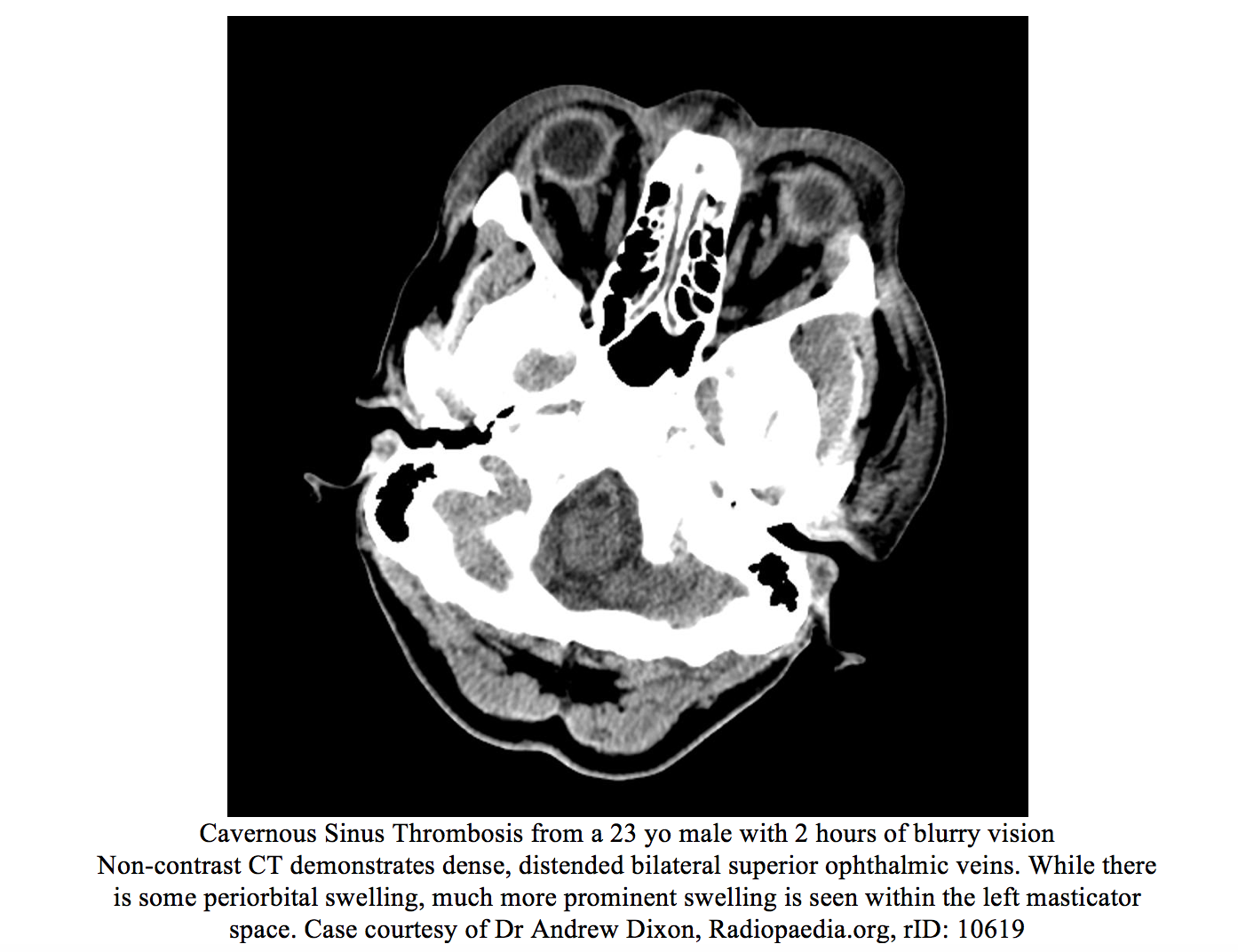
The first mimic is septic cavernous sinus thrombosis (CTS). Prior to antibiotics, septic CTS was nearly 100% fatal. CTS is now around 20-30% fatal if recognized and therapy is initiated in a timely manner. [7] The cavernous sinuses are uniquely situated. They are reflections of the dura matter that lie on either side of the sella turcica and the pituitary. The dura splits in to a trabeculated cavernous pattern and is separated by the thin bone of the sphenoid sinus. There are no valves, and thus blood flows freely. The cavernous sinus receives blood from the superior ophthalmic, cerebral veins, and sphenoparietal sinuses. The cavernous sinus then drains into the transverse sinuses and internal jugular veins. It also communicates with the pterygoid plexus, which communicates with the inferior ophthalmic vein and deep facial veins. The cavernous sinus houses several important structures that make it such a critical structure. Cranial nerves III, IV, V, and VI course through or along the outer edge the cavernous sinus. The cavernous sinus also contains the horizontal portion of the carotid artery. Dysfunction of any one of these structures can be a presenting feature of CTS. [8]
The cavernous sinus is uniquely set up for thrombosis and extension of infection from other sites. Sinusitis, especially sinusitis in the sphenoid and ethmoid sinuses is the most common cause of thrombosis. Less common are infections from other sites of the head and neck, including face, teeth, tonsils, nose, ears, eyes, etc. The most common symptoms of cavernous sinus thrombosis are due to dysfunction of the nerves and carotid artery passing through the sinus. Typically, these include headache (often a frontal headache), ophthalmoplegias (CN III, IV and VI), pupillary defects (CN III), sensory deficits of the face (CN V), vision changes due to involvement of ophthalmic veins, and stroke-like symptoms with involvement of the carotid artery. It may also spread by direct extension to the pituitary, meninges, or brain and present with features of these infections. [9] [10]

Clinically the presentation of CTS can be variable. It can present both acutely with rapid onset of symptoms or take a more subacute course over several days. Patients will almost always develop fever, proptosis, chemosis, ptosis, and ophthalmoplegia. Ophthalmoplegia may be variable initially. Usually patients will present with a lateral rectus (CN VI nerve) palsy, then progress to palsy of cranial nerves III and IV. Within 24-48 hours the disease will spread to the opposite eye and is a distinguishing feature of CTS. Early findings are typically headaches, tearing, violaceous edema of the upper eyelid, and periorbital swelling. Late findings include papilledema, retinal vein engorgement, and lethargy or obtundation. Visual disturbances, internal-ophthalmoplegia (involvement of the parasympathetic fibers of CNIII, or ciliary nerves), and trigeminal sensory changes occur in less than 50% of cases. Patients may have large or small pupils depending on the pattern of involvement. Less common are diplopia, seizure, and hemiparesis. Strokes are less common, but usually present as neuro deficits from direct extension of the infection into brain parenchyma, or from extension into the dural sinuses resulting in venous strokes. [9] [10]
The most common condition to mimic CST is orbital cellulitis which presents with proptosis and painful ophthalmoplegia. CST differs from orbital cellulitis by extension to the contralateral side, papilledema, pupillary defects, decreased periocular sensation. CSF in CTS may show isolated intracranial hypertension, lymphocytic pleocytosis, elevated RBC’s, and elevated protein. [11] [12] Orbital apex syndrome results from direct extension of sinusitis into the superior orbital fissure and affects CN III, IV, and VI. It is differentiated by acuity loss and ophthalmoplegia out of proportion to anterior orbital signs like proptosis and periorbital edema. The visual loss is more common than in CTS. [13]
Prior to CT and MRI, CTS was diagnosed by clinical presentation or by angiography. Angiography is no longer used due to being difficult in performing and high complication rate. [14] Data is not definitive on which modality is more accurate, but high-resolution CT with 3mm slices is used more frequently because of its ease of access and rapid assessment. Coronal images on CT will show flattening of the lateral wall of the cavernous sinus or convexity from the increased pressure within the sinus. Irregular filling patterns within the sinus are suggestive of thrombus. Indirect findings on CT include superior ophthalmic vein dilation, exophthalmos, soft tissue edema, and thrombi visualized in the veins that drain into the sinus. [15] MRI is beneficial for repeat assessment, or for evaluation of the meninges, brain tissue, or pituitary necrosis from direct extension. [16]
Management focuses on treatment of the underlying infection from the primary site. This includes covering for things such as sinusitis, dental abscesses, meningitis Antibiotics to consider include Nafcillin, ceftriaxone, metronidazole, cefotaxime or other antibiotics with similar coverage of head and neck pathogens. Vancomycin should be considered if methicillin resistant staphylococcal aureus (MRSA) is suspected. [8] Duration of treatment has not been studied, and no definitive time course exists. Consensus leans towards treating for 3-4 weeks like other endovascular infections. Surgical options are limited and are usually reserved for source control of the primary site of infection. Data for corticosteroids is sparse, but they may help patients with adrenal insufficiency related to pituitary necrosis. [8] There exists some retrospective data related to anticoagulation with heparin. Patients may have improvement in cranial nerve dysfunction, and may have a mortality benefit. [17]
Brain Abscess
Another ‘can’t miss diagnosis’ is brain abscess. Brain abscesses are a relatively rare infection more common in immunocompromised patients. It can have significant morbidity and mortality and may result from several different types of infections and pathogens. Most often these occur in patients with HIV, immunocompromise from organ transplant, or recent surgical procedures. Most cases are from contiguous spread, and less than one third are hematogenous in origin. Most of cases in solid organ transplant patients are fungal. Nocardia is also common among the transplant population. HIV patients are more likely to have toxoplasma or tuberculosis. [18] Patients with recent neurosurgical procedures are predisposed to S. aureus or S. epidermidis. Infections related to contiguous spread include streptococcal species and anaerobic infections. Patients with endocarditis or pulmonary infections with arterio-venous fistulas have a higher likelihood due to embolic disease. [19]
Pathologically, the infection starts with a localized cerebritis that leads to perivascular inflammation, necrosis, and edema. As the infection progresses, fibroblasts and neovascularization essentially create a shell around a necrotic center. [20]
Clinically abscesses present with headache, fever, and altered mental status. Presentation can vary based on the location of the abscess. [19] Occipital lobe abscess may have visual changes, while infections of the motor or sensory pathways or cortex may present with motor and sensory deficits. Frontal lobe abscesses may cause personality changes or altered mental status. Abscesses in the brain stem may involve cranial nerves and cause obstructive hydrocephalus resulting in headache. Cerebellar involvement may result in discoordination or gait abnormalities. Seizures are also another common presentation. [21] Presenting symptoms of a brain abscess may initially be very subtle and progress as the abscess develops. [22]
The differential diagnosis is broad but generally includes head and neck infections such as bacterial meningitis, stroke, epidural abscess, subdural empyema, and brain tumors. In HIV patients, lymphoma is also a consideration. [22]
The first diagnostic test of choice is Computed Tomography (CT) with contrast and should be performed in all patients suspected of brain abscess. [22] MRI has a high positive predictive value and is useful to differentiate an abscess from tumor. [23] Lumbar puncture and CSF culture are of high yield in these patients. CSF cultures are positive approximately 25% of the time. This needs to be weighed against the risk of brain herniation if symptoms of hydrocephalus are present. Lumbar puncture is generally reserved if there is clinical suspicion for rupture of the abscess into the ventricular system or suspicion for meningitis. Any head infection such as sinusitis, dental infections, and ear infections should be cultured and source control obtained. Neurosurgery should be involved early in the diagnostic course of brain abscesses. [19]

Meningitis
Meningitis is a tricky mimic given the way it overlaps with many other diagnoses. Fortunately, bacterial meningitis is not as common of a diagnosis as it previously once was in developed countries due to the effectiveness of vaccinations. It is up to 10 times more common in developing countries. [25] Vaccinations for Haemophilus influenzae type B and Streptococcus pneumoniae have been the most effective in reducing invasive bacterial CNS infections. Since the approval of vaccinations for children against Neisseria meningitidis, the peak incidence of has shifted from children to adults. [26] Currently the two most common agents in adults are S. pneumoniae and N. meningitidis accounting for up to 80% of cases. The overall incidence of pneumococcal meningitis has dropped sharply because of the pneumococcal vaccine. This has resulted in change in the predominant pneumococcal serotypes. Serotype 19A is now the most common pneumococcal serotype since the induction of the 13-valent pneumococcal conjugate vaccine. [27]
Bacterial meningitis occurs via two mechanisms. The first is hematogenous spread, especially common in neonates, and the second is direct extension from an infected site. The sites most common for direct spread are the sinuses and otitis media, although this is less common since the advent of the pneumococcal vaccine. Other infections such as a brain abscess can extend to the meninges. Patient typically at higher risk are those with infections in the ears, sinuses, those with recent neurosurgical procedures, or who have implants. Other patients at risk are the immunocompromised, endocarditis patients, diabetics, and alcoholics. [28]
Typically, meningitis is classified by the duration of its presentation. Acute meningitis is typically bacterial and presents in 24 hours or less from symptom onset. Subacute meningitis may present from 1-7 days and is more likely to be viral; however, a significant subset of these are bacterial. Chronic meningitis is anything presenting > 1 week. These chronic cases are more likely from causes such as TB, Syphilis, fungal, or carcinoma. The “classic” triad of fever, neck stiffness, and altered mental status is present in around 2/3 of patients, making these findings unreliable for diagnosis. Almost all patients will present with at least 2 of the following: headache, Fever, neck stiffness, and altered mental status. They may also present with other nonspecific symptoms including photophobia, viral prodrome, vomiting, seizures, or focal neuro deficits. This makes the clinical diagnosis even more tricky given these symptoms are all nonspecific. And when you consider that meningitis often results from direct extension from the sinuses, symptoms such as headache become more difficult to differentiate. [29]
The physical exam can be even less useful. Classic clinical tests have poor sensitivity. Nuchal rigidity has a sensitivity of 13%, Kernig’s 2%, and Brudzinski’s 2%. These tests may be more specific when present (Kernig’s 97%, and Brudzinski’s 98%), however excluding meningitis based on the lack of these findings is a fool’s errand. [25] The Jolt test, has been touted as being 100% sensitive, but recent evidence suggests that it is not as sensitive as previously believed and should not be used solely to rule in or rule out meningitis. [30]
Work up for meningitis includes chemistries, CBC, blood culture, classically a CT head prior to LP (recent evidence shows CT is not needed in neuro intact patients with normal mental status), a CXR given 50% of pneumococcal meningitis patients have a pneumonia, and a lumbar puncture. An LP can be safely done if the patient is < 60 years, is without immunocompromise, has no history of prior CNS disease, and has not had a seizure within the last week prior to presentation. If a CT is done, LP should not be performed if there is a midline shift, obstructive hydrocephalus, compressed basilar cisterns, or a posterior fossa mass. LP interpretation is a discussion in and of its self. Findings of bacterial meningitis include cloudy or purulent CSF, opening pressure > 25 mm Hg, WBC count > 100, > 80% PMN’s, low glucose, and high protein, or + gram stain. If the tap is bloody, the counts of the LP may need to be corrected. Two hours after antibiotics, CSF cultures become negative for Neisseria, and after 6 hours for pneumococcal. After 12 hours, glucose and protein will start to normalize. [25]

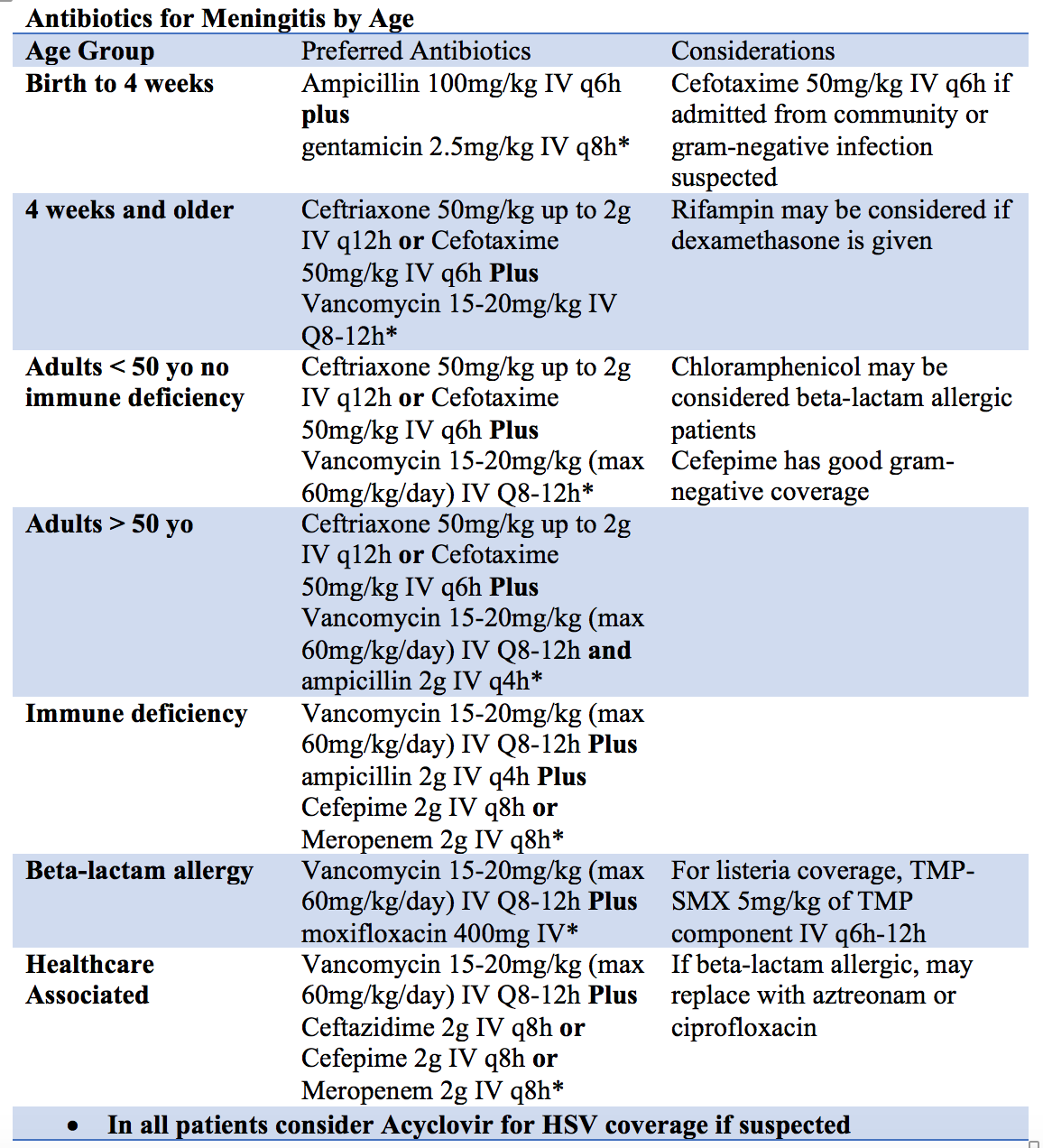
Antibiotic therapy should be tailored to age. Neonates to 1 month are at higher risk for Listeria and genitourinary flora. Ampicillin + cefotaxime or gentamicin are a good combo. HSV is usually considered due to the higher risk for HSV in this age group. Vancomycin is also considered if S. pneumonia or MRSA is suspected. For patient’s > 1 month old, ceftriaxone + vancomycin is recommended. If an adult is > 50 and immunocompromised, ampicillin should be considered since listeria starts to work its way back into the mix. If there was a recent surgical procedure in the CNS, Cefepime and Vancomycin are considered. In penicillin allergic patients, consider chloramphenicol + vancomycin as an alternative. For Neisseria prophylaxis, Ceftriaxone, ciprofloxacin, or Rifampin are commonly used. [31]
Steroids have been shown to have a mortality benefit in adults. This is not true of children. Dexamethasone is usually given 15 minutes prior to antibiotic for greatest benefit, and then dosed every 6 hours thereafter. [32]
Prophylaxis should be given for people who live with the patient, school contacts, or daycare contacts within the last 7 days; if they have had direct exposure to a patient’s secretions; or for the provider who intubated without a facemask. These patients should be admitted with droplet precautions. [33]
The significant overlap in fever and headache, and the pathophysiology of meningitis compared to sinusitis especially within the first 24 hours makes this a challenging diagnosis even for the best of physicians.
Orbital/ Periorbital Cellulitis
Orbital cellulitis is another diagnosis closely linked to sinusitis. It is a true emergency and requires a high index of suspicion and a conservative approach. Most commonly it results from direct extension of a local infection. Usually this is from the ethmoid sinus. Other sites and causes implicated are trauma, dental infections, ear infections, and endophthalmitis. Orbital cellulitis can be difficult to distinguish in its early course due to its similarity with periorbital cellulitis. Orbital cellulitis and periorbital cellulitis differ in the location of the infection. Orbital cellulitis is an infection behind the eye within the orbit. Periorbital cellulitis is anterior to the eye in the soft tissue and palpebrae. The two locations are separated by a fascial plane. [34]
Orbital cellulitis and periorbital cellulitis both present similarly early with swelling and erythema of the soft tissues surrounding the orbit. Orbital cellulitis will then progress to involve the posterior structures of the orbit. Commonly the eye may be proptotic and the globe displaced. Vascular congestion and inflammation may lead to chemosis of the affected eye. Compression and inflammation of the orbital muscles leads to limited and painful eye movements, which may result in double vision. Decreased visual acuity occurs from compression and inflammation surrounding the optic nerve. [34] [35] [36]
The differential of orbital cellulitis and periorbital swelling is based on if the eye is proptotic or not. If the eye is proptotic, then the question becomes is there increased intraocular pressure? Diagnoses with a normal intraocular pressure include cellulitis, orbital pseudotumor, or orbital tumors. If the eye pressure is elevated, retrobulbar abscess, retrobulbar hemorrhage, retrobulbar emphysema, or an ocular tumor are likely. If there is no proptosis, then the diagnosis is likely preseptal in nature and is likely related to periorbital cellulitis, dacryocystitis, dacryoadenitis, allergic reaction, or nephrotic syndrome. Complications of the eye lid can also lead to periorbital swelling, such as a chalazion or stye. Other considerations include subperiosteal abscess, cavernous sinus thrombosis, conjunctivitis, dermatitis, zoster infections, and granulomatous disease. The clinical feature that has significant overlap with many of these diagnoses is a frontal headache. [34] [35]
If clinically suspected based on history and physical exam, the evaluation should include a CT of the orbits with IV contrast. There are no labs or markers that are sensitive or specific enough to differentiate. Findings on imaging include proptosis, inflammation of ocular muscles, and a defined orbital abscess or subperiosteal abscess. [34] [37]

Therapy should be aimed at flora of the head, pharynx, and sinuses. Vancomycin is the anchor of this regiment due to the high prevalence of penicillin resistant gram-positive organisms. In addition to vancomycin, a second agent such as ampicillin-sulbactam, ceftriaxone, piperacillin-tazobactam, should be used. If suspicion for fungal infection or in a diabetic patient, Amphotericin B is a consideration. These patients should be immediately evaluated by ophthalmology and admitted for antibiotics and surgical interventions. [34] [37]
Mucormycosis
Immunocompromised patients deserve a special consideration when it comes to sinusitis as this group is at higher risk for invasive fungal infections like mucormycosis. Diabetics are at high risk for this condition due to hyperglycemic conditions, providing optimal growth for the organism. Mucor is found in the environment and commonly is the mold in bread and fruit. It is spread by spores dispersed in the air, entering the host via nasal passages. It most commonly invades through the local vascular system into the sinuses and brain. It can also involve other sites including the lungs, GI tract, and skin. [36] [38]
Initially the condition presents like acute sinusitis and can lead to swelling of the cheeks and periorbital tissues. From there it spreads locally and rapidly to the orbits, sinuses, vasculature, and brain. Clinically it can cause vision changes from vascular thrombosis, nasopharyngeal, oropharyngeal, ulceration and eschar, facial swelling, and headache. Commonly the mucosa of infected areas become necrotic from local thrombosis and vascular invasion and appear black. [39] [38]
Treatment is emergent ENT consultation for debridement of infected tissue. This is the definitive treatment. Patients should also be started on aggressive antifungal therapy with amphotericin B. They should be aggressively resuscitated and intubated early if at risk for airway involvement. Iron chelation (mucor loves iron, avoid deferoxamine as this can increase iron uptake in mucor and be counterproductive) can be considered but should usually be done in conjunction with ID specialists and may not be readily available in the ED. Hyperbaric oxygen is also a consideration. [40] [41]
Nasal foreign bodies
Kids may present with persistent congestion rather than the headache and facial pain common in adults. Much of this is due to the developmental anatomy of the sinuses, as they are not present at very young ages. Children are known to put anything in their nose. Typically, they present with unilateral nasal congestion that is persistent and may be foul smelling, or they may present with unilateral features of sinusitis. [42]
Removal of a nasal foreign body can be a challenge and may require procedural sedation. If the foreign body is difficult to locate and suspicion is high, radiographs may be helpful in diagnosis. It is especially important to have a high index of suspicion for magnets or button batteries as these will erode through the septum. Where there is one foreign body, there may be more and a thorough exam of the contralateral nares and ears, and consider examining the GU area in females as well. [42]
Before removal, oxymetolazone or aerosolized lidocaine can assist. There are multiple techniques for removal. These techniques are a complete discussion separately and will not be discussed in detail here. Techniques include suctioning, balloon catheter or foley catheter, Dermabond on a cotton tip applicator shielded by a nasal speculum, a lighted curette, Alligator forceps, “Parent’s Kiss,” or a strong magnet. Each technique has its risk and should be used in as safe a manner as possible. Usually the optimal position is to perform the foreign body removal with the patient in the upright position. ENT should be consulted if removal proves unsuccessful, or if there are complications. [42]
Dental infections
Among the most common of the mimics includes dental infections and dental trauma. Dental problems can cause pain and mimic sinusitis by direct extension of infection or trauma into the sinuses, extension into the local and cutaneous structures of the face, or by referred pain from shared innervation.
There are many different reasons for dental pain and is a whole discussion by itself. In infants, tooth eruptions may result in pain and a low-grade fever in 12% of children. ABS in this age group is very uncommon due to the lack of developed sinuses. Tooth eruptions in older children can result in gingival irritation and referred pain. This can also present similarly to pericoronitis which is inflammation of gingival tissue overlying the occlusive surface of the erupting tooth and can be a serious life-threatening infection. If the 3rd molar is involved, there may be extension into the masticator space causing trismus. Generally, these infections are treated with penicillin and if severe, may require IV antibiotics. These patients should be referred to a dentist. [43]
Dental Caries are incredibly common. They are the result of tooth erosion from acid producing bacteria in plaques. Usually these plaques are located along the occlusal surface of the tooth. After erosion through the enamel, the bacteria then spread throughout the dentin of the tooth, and exposure to the environment results in pain. The resultant inflammation within the pulp of the tooth is called pulpitis and may be reversible depending on how long the symptoms are present. If pain is present for seconds to minutes, then likely it is reversible. If present for hours then likely not. Treatment is straightforward, NSAIDS and/or a dental block for pain control, antibiotics should be reserved for cases with clear evidence of abscess or infection. [44] [45] Nerve blocks are a good consideration for temporary pain relief in these patients. Complications of carries include spread beyond the tooth resulting in peri-radicular periodontitis apical abscesses and further including to the sinuses and face. Treatment remains the same as for caries, Penicillin or clindamycin, pain control and dental referral for definitive treatment. [43]

Spread into the facial spaces are common complications from dental infections. Infections of the lower teeth spread to the buccinator space where as infections from upper teeth tend to spread to the maxillary and infraorbital spaces. Infections from the lower molars have potential to spread along the mandible to the lingual space, and from there to the submandibular space resulting in a deep space infection better known as Ludwig’s angina. These spaces communicated with the pharyngeal space and extension of the infection into the pharyngeal space can lead to airway compromise. Infection in the infraorbital space can lead to direct extension to posterior structures and lead to other serious infections such as cavernous sinus thrombosis. [43]
Brain Tumors
Sinusitis loves to present with headache, specifically frontal headache. One consideration to keep in the back of your mind are CNS tumors. These tumors can present in a varied fashion including headache that is usually generalized or occipital, but they can present in a varied fashion including a frontal headache. The classic presentation is a headache that is worse in the morning and improves with being upright through the day. Headache may also present as tension or migrainous type headache in up to half of patients. [46] Neurologic symptoms are clinically what raises concern for a CNS tumor. Any presentation of headache with another neurologic feature should raise concern. These include focal neurologic deficits, seizures, altered mental status, and something as simple as difficulty concentrating. In kids, be highly suspicious if there is vertical nystagmus or ataxia. Brain tumors in children usually present in the posterior fossa and will present with elevated ICP, hydrocephalus, macrocephaly, or cerebellar findings. [47] [48]
Because the presentation can be so subtle, evaluation can be quite challenging. Brain tumors don’t have serologic markers to help with screening. Markers like an erythrocyte sedimentation rate are going to be low yield. If you have a high index of suspicion MR of the brain is really the test of choice. Specifically, MR with contrast because many tumors have some necrosis with surrounding edema which will light up with contrast on the scan. CT is much less sensitive and should be a backup test for patients unable to undergo MR. There really is no benefit to LP, EEG, or angiography when looking for a tumor. [47]
If the tumor is symptomatic, the goal is to reduce edema immediately. This is done by giving glucocorticoids, usually dexamethasone with initial bolus of 10mg followed by 4mg 4 times per day. [49] Anticonvulsants are a consideration if there are seizures, but generally are not used for prophylaxis. Definitive treatment usually consists of surgical intervention, chemotherapy, or radiation. A neuro surgeon should be brought on board early, especially if there are symptoms of elevated ICP, or conversion to hemorrhage. [50]
Conclusion
Looking back at our original case, what is likely to be going on with this patient? He has multiple findings of concern. First, he is a bounce back for similar symptoms. He has progression with new symptoms including headache, fever and double vision. On exam, there is a pupillary defect, extraocular muscle involvement and proptosis. Given this, out likely diagnoses would be orbital cellulitis vs cavernous sinus thrombosis. A CT head and orbits with contrast was done and revealed an abscess of the retroorbital space because of direct extension from the ipsilateral ethmoid sinus. The patient was started on IV antibiotics and taken for surgical drainage by ENT and ophthalmology. He did well and after several days was discharged home to complete a course of antibiotics. This case was not a formal case report, but a patient I had personally taken care of. It illustrates well just how much overlap there can be between a simple sinus infection and multiple pathologies and how one can evolve into the other. It is crucial when evaluating these patients to recognize how interrelated these different disease processes are and to always have a high index of suspicion.
Take home points:
- Sinusitis symptoms overlap with many other diseases, only some of the prominent diseases were covered here.
- CTS presents with unilateral retroorbital and frontal headache and cranial nerve deficits. CTS and sinusitis can occur concurrently. Include a detailed ocular and neurologic exam in your assessment to help clue you in.
- Brain abscesses can result as a direct extension of sinusitis. Headache is a common presenting feature, which may be frontal or retroorbital. Fevers are also common to both. In patients with sinusitis, consider a brain abscess if they have mental status changes, lethargy, or subtle or progressive neurologic deficits.
- Meningitis presents with headache and fever and can mimic sinusitis. Be wary of sinusitis directly extending to the CNS. Physical exam is unreliable for ruling out meningitis. Maintain a high index of suspicion in sinusitis patients that have other symptoms suggestive of meningitis.
- Orbital cellulitis often occurs as a direct extension of sinusitis. Be suspicious when patients have vision complaints and ocular findings on exam including proptosis and antalgic eye movements. These often require surgical management
- Mucormycosis is a surgical emergency! Always do a thorough oral and nasal exam to look for necrotic tissue and black eschar in the immunocompromised and diabetics
- Think nasal foreign body in a kid with unilateral congestion. Be sure to check all orifices as kids like to put things everywhere.
- Headache with positional changes or progression can be a brain tumor. Be sure to evaluate for these signs in a sinusitis patient.
This post is sponsored by www.ERdocFinder.com, a supporter of FOAM and medical education, who with their sponsorship are making FOAM material more accessible to emergency physicians around the world.
References/Further Reading:
| [1] | Benninger, “Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology,” vol. 129, no. 3, pp. S1-S32, September 2003. |
| [2] | R. M. Rosenfeld, “Clinical Practice Guideline (Update): Adult Sinusitis,” Otolarynology-Head and Neck Surgery, vol. 152, no. 2, pp. S1-S39, 1 April 2015. |
| [3] | E. R. W. Gregory DeMuri, “Acute Bacterial Sinusitis in Children,” Pediatrics in Review, vol. 34, no. 10, pp. 429-437, Oct 2013. |
| [4] | W. Fokkens, “Avoid Prescribing Antibiotics in Acute Rhinosinusitis,” British Medical Journal, vol. 349, no. g5703, 2014. |
| [5] | J. Bird, “Adult Acute Rhinosinusitis,” British Medical Journal, vol. 346, no. f2687, 2013. |
| [6] | A. Chow, “IDSA Clinical Practice Guideline for Acute Bacterial Rhinosinusitis in Children and Adults,” Clinical Infectious Diseases, vol. 54, no. e12, 2012. |
| [7] | J. Clune, “Septic Thrombosis within the Cavernous Chamber,” Am Journal Ophthalmology, vol. 56, pp. 33-39, 1963. |
| [8] | J. Ebright, “Septic Thrombosis of the Cavernous Sinus,” Arch Intern Med, vol. 161, pp. 2671-2676, 2001. |
| [9] | M. DiNubile, “Septic Thrombosis of the Cavernous Sinuses,” Arch Neurology, vol. 45, pp. 567-572, 1988. |
| [10] | F. Southwick, “Septic Thrombosis of the Venous Dural Sinuses,” Medicine (Baltimore), vol. 65, pp. 82-106, 1986. |
| [11] | B. Biousse, “Isolated Intracranial Hypertension as the Only Sign of Cerebral Venous Thrombosis,” Journal ofNeurology, vol. 53, p. 1537, 1999. |
| [12] | C. Price, “Cavernous Sinus Thrombosis and Orbital Cellulitis,” South Med Journal, vol. 64, pp. 1243-1247, 1971. |
| [13] | E. Kronschnabel, “Orbital Apex Syndrome due to Sinus Infection,” Laryngoscope, vol. 84, pp. 353-371, 1974. |
| [14] | G. Brismar, “Aseptic Thrombosis of Orbital Veins and Cavernous Sinus,” Acta Ophthalmologica, vol. 55, pp. 9-22, 1977. |
| [15] | J. Ahmadi, “CT Observations Pertinent to Septic Cavernous Sinus Thrombosis,” American Journal of Neuroradiology, vol. 6, pp. 755-758, 1985. |
| [16] | J. Berge, “Cavernous Sinus Thrombosis Diagnostic Approach,” Journal of Neuroradiology, vol. 21, pp. 101-117, 1994. |
| [17] | S. Levine, “The Role of Anticoagulation in Cavernous Sinus Thrombosis,” Neurology, vol. 38, pp. 517-522, 1988. |
| [18] | I. Tan, “HIV-Associated Opportunistic Infections of the CNS,” Lancet Neurology, vol. 11, pp. 605-617, 2012. |
| [19] | M. Brouwer, “Clinical Characteristics and Outcomes of Brain Abscesses: Systematic Review and Meta-analysis,” Neurology, vol. 82, pp. 806-613, 2014. |
| [20] | R. Britt, “Neuropathological and Computerized Tomographic Findings in Experimental Brain Abscess,” Journal of Neurosugery, vol. 55, pp. 590-603, 1981. |
| [21] | Shaw, “Cerebellar Abscesses: A Review of 47 Cases,” Journal of Neurosurgery and Psychology, vol. 38, pp. 429-435, 1975. |
| [22] | M. Brouwer, “Brain Abscess,” New England Journal of Medicine, vol. 371, pp. 447-456, 31 July 2014. |
| [23] | J. Reddy, “The Role of Diffusion Weighted Imaging in the Differential Diagnosis of Intracranial Cystic Mass Lesions: A report of 147 Lesions,” Surg Neurol, vol. 66, pp. 246-250, 2006. |
| [24] | Guitierrez-Cuadra, “Brain Abscesses in a Third Level Hospital: Epidemiology and Prognostic Factors Related to Mortality,” Rev Esp Quimioter, vol. 106, pp. 201-206, 2009. |
| [25] | M. Fitch, “Emergency Diagnosis and Treatment of Adult Meningitis,” Lancet, vol. 7, pp. 191-198, 2007. |
| [26] | S. McIntyre, “Effect of Vaccines on Bacterial Meningitis World Wide,” Lancet, vol. 380, p. 1703, 2012. |
| [27] | L. Olarte, “13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis in US Children,” Clinical Infectious Disease, vol. 61, p. 767, 2015. |
| [28] | M. Durand, “Acute Bacterial Meningitis in Adults. A Review of 493 Episodes,” New England Journal of Medicine, vol. 328, p. 21, 1993. |
| [29] | D. e. a. van de Beek, “Clinical Features and Prognostic Factors in Adults with Bacterial Meningitis,” New England Journal of Medicine, vol. 351, no. 18, pp. 1849-1859, 28 Oct 2004. |
| [30] | J. Nakao, “Jolt Accentuation of Headache and Other Clinical Signs: Poor Predictors of Meningitis in Adults,” American Journal of Emergency Medicine, vol. 32, no. 1, pp. 24-28, 2014. |
| [31] | D. Van de Beek, “Advances in Treatment of Bacterial Meningitis,” Lancet, vol. 380, no. 9854, pp. 1693-1702, 10 November 2012. |
| [32] | M. Brouwer, “Corticosteroids for Acute Bacterial Meningitis,” Cochrane Database of Systematic Reviews, no. 9, 2010. |
| [33] | A. Chaudhuri, “EFNS Guideline on the Management of Community-Acquired Bacterial Meningitis: Report of an EFNS Task Force on Acute Bacterial Meningitis in Older Children and Adults,” European Journal of Neurology, vol. 15, no. 7, pp. 649-659, 2008. |
| [34] | A. Hauser, “Periorbital and Orbital Cellulitis,” Pediatrics in Review, vol. 31, no. 6, pp. 242-249, 2010. |
| [35] | T. Ekhlassi, “Preseptal and Orbital Cellulitis,” Disease-a-Month, vol. 63, no. 2, pp. 30-32. |
| [36] | A. Farooq, “Fungal Orbital Cellulitis: Presenting Features, Managment and Outcomes at a Referral Center,” Orbit, vol. 34, no. 3, 2015. |
| [37] | J. Bedwell, “Management of Pediatric Orbital Cellulitis and Abscess,” Current Opinion Otolaryngology Head and Neck Surgery, vol. 19, no. 16, pp. 467-713, December 2011. |
| [38] | M. Selvamani, “Mucormycosis Causing Maxillary Osteomyelitis,” Journal of Natural Science, Biology, and Medicine, vol. 6, no. 2, pp. 456-459, 2015. |
| [39] | H. Motaleb, “A Fatal Outcome of Rhino-orbito-cerebral Mucormycosis Following Tooth Extraction: A Case Report,” Journal of International Oral Health, vol. 7, pp. 68-71, 2015. |
| [40] | M. Mohamed, “Management of Rhino-Orbital Mucormycosis,” Saudi Medical Journal, vol. 36, no. 7, pp. 865-868, 2015. |
| [41] | F. Bellazreg, “Outcome of Mucormycosis After Treatment: Report of Five Cases,” New Microbes and New Infections, vol. 6, pp. 49-52, 2015. |
| [42] | R. Riviello, “Otolaryngologic Procedures,” in Roberts and Hedges’ Clinical Procedures in Emergency Medicine, pp. 1298-1341. |
| [43] | R. Beaudreau, “Oral and Dental Emergencies,” in Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, New York, NY, McGraw-Hill, 2016. |
| [44] | D. e. a. Nagle, “Effect of Systemic Penicilin on Pain in Untreated Irreversible Pulpitis,” Oral Surg Oral Med Oral Pathol Oral Radiol Endod, vol. 90, no. 5, pp. 636-640, 2000. |
| [45] | M. e. a. Runyon, “Efficacy of Penicillin for Dental Pain Without Overt Infection,” Academic Emergency Medicine, vol. 11, no. 12, pp. 1288-71, 2004. |
| [46] | P. J. Forsythe PA, “Headaches in Patients with Brain Tumors,” Neurology, vol. 43, p. 1678, 1993. |
| [47] | S. Weathers, “Tumors of the Central Nervous System,” in The MD Anderson Manual of Medical Oncology, 3e, New York, NY, McGraw-Hill. |
| [48] | T. Bouldin, “Pathology of the Nervous System,” in Pathology: A Modern Case Study, New York, NY, McGraw-Hill. |
| [49] | C. e. a. Vecht, “Dose-effect Relationship of Dexamethasone on Karnofsky Performance in Metastatic Brain Tumors: a Randomized Study of 4, 8, and 16mg Per Day,” Neurology, vol. 44, p. 675, 1994. |
| [50] | L. DeAngelis, “Primary and Metastatic Tumors of the Nervous System,” in Harrison’s Principles of Internal Medicine, 19e, New York, NY, McGraw-Hill, 2014. |
| [52] | M. Cannon, “Cavernous Sinus Thrombosis Complicating Sinusitis,” Pediatric Critical Care Medicine, vol. 5, no. 1, pp. 86-88, January 2004. |
| [53] | M. Arian, “Septic Cavernous Sinus Thrombosis: A Case Report,” Iran Red Crescent Medical Journal, vol. 18, no. 8, 19 June 2016. |
| [54] | M. Arian, “Septic Cavernous Sinus Thrombosis: A Case Report,” Iran Red Crescent Medical Journal, vol. 18, no. 8, June 2016. |
| [55] | I. Khatri, “Septic Cerebral Venous Sinus Thrombosis,” Journal of Neurologic Science, vol. 362, pp. 221-7, 15 March 2016. |
| [56] | K. Bhatia, “Septic Cavernous Sinus Thrombosis Secondary to Sinusitis: Are Anticoagulants Indicates? A Review of the Literature,” Journal of Larynology Otolarynology, vol. 116, no. 9, pp. 667-676, September 2002. |
| [57] | J. Carpenter, “Retrospective Analysis of 49 Cases of Brain Abscess and Review of the Literature,” European Journal of Clinical Microbiology and Infectious Diseases, vol. 26, pp. 1-11, 2007. |
| [58] | B. Schuknecht, “Tributary Venosinus Occlusion and Septic Cavernous Sinus Thrombosis: CT and MRI Findings,” American Journal of Neuro Radiology, vol. 19, pp. 617-626, 1998. |









