Author: Scott McAninch, MD, FACEP (@MacAttackSM, EM Attending Physician, Baylor Scott and White Memorial Hospital and McLane Children’s Hospital) // Edited by: Alex Koyfman, MD (@EMHighAK) and Brit Long, MD (@long_brit)
Case 1 Continuation
Your minimally responsive patient with blood-soaked clothes is carried to an ED room in your non-trauma designated hospital. He appears “sick”, and you anticipate the eventual need for a higher level of trauma care. You calmly ask your secretary to mobilize the transport team and call the trauma surgeon at the nearest trauma center for transfer acceptance (because it may take “a while” to get him/her on the phone). Your ED resuscitation team is made up of two new graduate ED nurses and a respiratory therapist. You calmly verbalize the situation and team goals, “Our patient is bleeding and sick. We need to control bleeding sources, stabilize other life-threatening conditions, and prepare for transfer to the trauma center as soon as possible.” Your team adopts your projection of tranquility and poise. The team asks you, “What do we do first?”
Introduction
Part One discussed creating and maintaining a calm, orderly, and relatively quiet resuscitation environment that is goal-oriented to complete life-saving tasks. Part two will discuss the clinical care of the “sick” trauma patient in the non-trauma center and suggestions for trauma process improvement. Clinical care includes early activation of transfer process, prioritized trauma survey physical exam, and application of “damage control resuscitation” treatment principals, as feasible in the non-trauma designated center.
Damage Control Resuscitation (DCR)
Severely-injured trauma patients may be in a shock state and develop acute coagulopathy of trauma. Coagulopathy, acidosis, and hypothermia are collectively termed the “lethal triad” of trauma. Resuscitation with aggressive IV crystalloid boluses is associated with worsening of the lethal triad, increased wound bleeding from intravascular hydrostatic pressure, coagulation factor dilution, abdominal compartment syndrome (1), and increased post-operative mortality (2,3). There is suggestion that as little as >500cc of crystalloid may increase mortality (4).
As an alternative to aggressive IV crystalloid infusion, damage control resuscitation (DCR) is a treatment strategy whose goal is to combat the lethal triad until the patient may receive damage control surgery (DCS) or definitive bleeding control. Combating the lethal triad is achieved by controlling hemorrhage sources; minimizing crystalloids; restoring vital organ perfusion with limited IV fluids, preferably balanced blood products, but permitting the patient to be temporarily hypotensive if vital organs are perfused (permissive hypotension); and preventing hypothermia (5,6). Some DCR measures may be employed in the non-trauma center, such as controlling external hemorrhage and preventing hypothermia. Other DCR principles, such as IV fluid resuscitation, are dependent upon resources and time to trauma center intervention. Permissive hypotension may have limited application, as discussed below.
Initiate trauma transfer procedure as soon as possible
In part one, we discussed suggested guidelines for transfer to the trauma center (7,8). As soon as you have identified the need for trauma transfer, your resuscitation goals should be “beast mode” focused: provide the essential, life-stabilizing care by damage control resuscitation principles and expedite transfer.
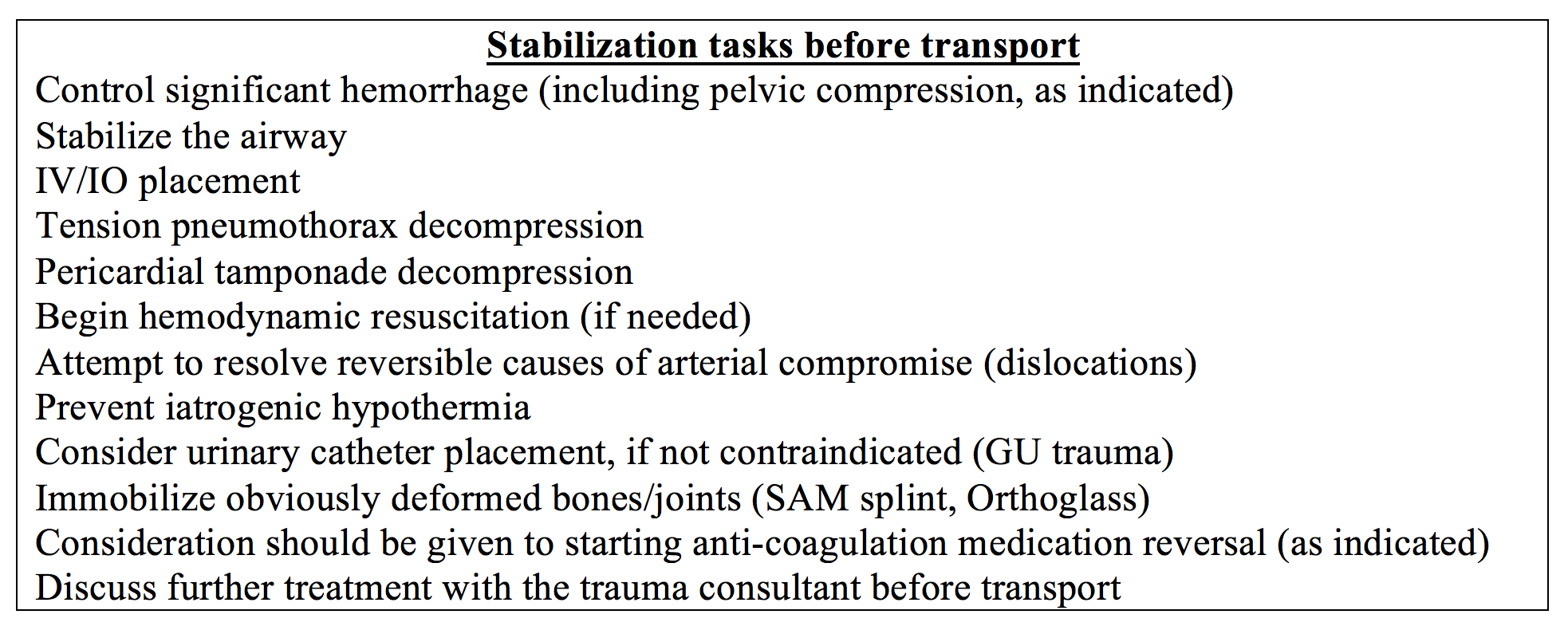
Call as soon as possible for both trauma center transfer acceptance and transfer transportation team (request they bring a portable ventilator if intubation anticipated). The accepting trauma provider may provide clinical guidance for ongoing resuscitation. Anticipate the trauma center asking questions related to their trauma “code” activation or mass transfusion criteria, such as mechanism of injury, vital signs (have them updated and available to you), history of blood thinner medications, etc.
Patient history
Obtain a “SAMPLE” history. If pre-hospital scene pictures are available (MVC demonstrating severe car damage), consider sending them to the trauma center as permissible by HIPAA and your hospital policy. Be alert for red flags indicating possible medical causes of the trauma (single vehicle incident with minimal trauma). Are there medications that may signal significant underlying medical problems or predispose to complications? Beta blockers (blunted tachycardia compensatory response), anticoagulants, factor replacement (hemophilias), DDAVP (Von Willebrand disease), fludrocortisone or hydrocortisone (adrenal insufficiency), or immunosuppressants (inflammatory conditions, transplant anti-rejection)?
Primary physical examination
Primary trauma survey priorities are well-known by the ATLS “ABCDE” model. However, significant external hemorrhage (“C”) should be controlled before proceeding to airway (“A”), as modeled in Tactical Combat Casualty Care (9):
Circulation/External hemorrhage
If arterial hemorrhage is present, you may attempt direct pressure control with hemostatic gauze, but often an extremity tourniquet(s) is needed to stop bright red bleeding. If no tourniquet is available, use a blood pressure cuff or other removable constricting device, preferably with a wide-diameter (to avoid further soft tissue damage). Document placement time. Attempt to remove within 2 hours (9), although six hours may be safe (10). Patients often need pain control. If bleeding is significant in a “junctional” area (axilla, groin), or otherwise non-arterial in any other area, use direct pressure and fill-in tissue cavities with hemostatic gauze to tamponade the wound. Avoid thick circumferential layers of gauze, as they reduce ability to apply direct pressure to the wound. Don’t “explore” the wound unless directed by the trauma consultant. Some wounds that are initially stable may begin to bleed later as you resuscitate with IV fluids.
Airway
If you have concern for airway compromise before the patient will arrive at the receiving trauma center, then consider placing definitive airway before transfer. Pre-oxygenate early and consider apneic oxygenation (11). Consider reversible causes of respiratory insufficiency (hypoxia, hypoglycemia, opioids). “Delayed sequence intubation” (DSI) (12) may be considered for patients who are difficult to pre-oxygenate due to altered mentation. Consider improving shock index (13,14) to avoid post-intubation complications related to hemodynamic instability (15,16). Both etomidate and ketamine may be used as induction agents in adults (17-19) and pediatrics (20,21). Obtain a succinct neurologic examination (your “D” exam) before DSI/RSI, if possible. Prepare a “triple set up” intubation: endotracheal (may use cuffed ET tubes in children >3kg), supraglottic and cricothyroidotomy (needle cricothyroidotomy if < 8-10 years old (22)). Have a “bougie” nearby. If applicable, maintain cervical spine immobilization while the C collar is removed during intubation. Infant ET tubes may displace easily with head rotation. Ask the transport team to bring a ventilator. Be prepared to manage the ventilator. “Spinning the Dials” is a great resource for ventilator management in adult patients (23). Place an orogastric tube to decompress stomach, especially in infants, to allow for better ventilation.
Breathing
If there is an open pneumothorax (PTX), cover it and perform a tube thoracostomy in another appropriate same-side site. Decompress moderate to large (tension) closed PTX’s. If the patient will be going up in altitude during transfer with any size PTX, consider decompression before transfer (as the PTX may expand with increasing altitude) (24). Of note, angiocatheters used in needle decompression of PTX may become occluded during transport, so consider performing a tube thoracostomy before transfer. If pulmonary contusions are suspected, limit IV fluids as feasible.
Circulation (other than external hemorrhage above)
Place IV or IO (avoid growth plates in children). Two “large” bore IV’s are preferable. Intranasal fentanyl (1.5 micrograms/kg) in children may ease IV/IO placement. Send blood for point of care and laboratory tests. Perform E-FAST (including quick transabdominal assessment for pregnancy, if applicable) before “rolling” the patient. Relieve cardiac tamponade. Check for signs of arterial compromise and any correctable causes (dislocated joint, compartment syndrome from burns). If you have concern for pelvic space hemorrhage, place pelvic ring compression (pelvic binder, bedsheet tied). DCR fluid resuscitation strategies are discussed below.
Disability
Perform before you RSI so trauma center personnel know patient’s baseline for ongoing neurologic assessments. Pupil responses should not be effected by paralytic meds (25).
Exposure/Environment
Remove clothing and place in evidence bags, as indicated. Look, listen, smell, and feel. While maintaining any spinal precautions, examine the back and hidden areas (axilla, groin, perineal, intergluteal). Small ammunition and narrow weapon (ice picks) wounds may be deceivingly small. Secure position of penetrating foreign bodies. After clothing is removed, prevent hypothermia, especially in those with decreased heating mechanisms (infants, elderly). Hypothermia is associated with acute coagulopathy of trauma (26). You can provide warm blankets, warm humidified air, warmed IV fluids to a target of 40-42C. You may use a microwave or rapid fluid infuser to warm crystalloid fluids. PRBC’s and FFP may be infused through an IV fluid warmer (Level One, Ranger) to mitigate hypothermia. Do not microwave blood products.
Foley
A Foley (or appropriate urinary catheter, if no contraindication) may relieve angst and agitation of patients who have a diuretic, caffeine, or alcohol on-board. Also, urinary output is used to estimate renal blood flow and adequacy of resuscitation. Send a urine sample for urinalysis, urine pregnancy test, and urine drug screen, as needed. Suprapubic bladder aspiration, preferably ultrasound guided, may be used in extreme circumstances.
Who needs IV fluid resuscitation?
IV fluid resuscitation is a controversial and changing landscape. At the very least, IV fluid strategies should be considered for patients in hemorrhagic shock. But, how do we determine who is in hemorrhagic shock? The ATLS model offers a framework of variables for shock, but its ability to correlate to actual blood loss has been questioned (27,28). Currently, one may consider the combination of vital signs, physical exam findings, and mass transfusion protocol triggers, including some diagnostic tests to help determine who may be in a shock state.

IV Fluid Resuscitation for hemorrhagic shock (and no TBI)
Trauma patients in hemorrhagic shock with vital organ compromise warrant IV fluid resuscitation. Selected patients demonstrating shock but with adequate vital organ perfusion may be considered for a “permissive hypotension” strategy until surgical intervention is obtained. Be aware other etiologies of shock may be present and treated accordingly (neurogenic shock, septic shock, etc.). IV fluid strategies should be discussed with the trauma surgery consultant.
Shock and vital organ compromise
The DCR IV fluid resuscitation approach uses balanced blood product replacement and minimal to no crystalloids with the immediate goal of restoring vital organ perfusion and SBP >70mmHg (3). Although MTP is not available at many centers, EAST guidelines and ATLS TQIP support MTP use with immediate balanced blood product resuscitation if MTP triggers are met. (32,38). In the absence of a MTP protocol, emergency release type O blood may be given initially, and if other blood products are available, they may be administered in a balanced ratio of 1:1:1 (6 units PRBC, 6 units FFP, 1 apheresis unit/6 pack of random donor platelets) (39). If only PRBC and FFP are available, a 1:1 ratio is preferable (40). If blood is not immediately available, consider small crystalloid boluses until ready. Warm PRBC’s and FFP as possible.
Alternatively, if no blood products are available, the ATLS resuscitation model uses an IV crystalloid 1-2L challenge to assess for need of blood product replacement. (41) Balanced crystalloid solutions (Lactated Ringers, Plasmalyte) may be preferred over normal saline (NS) (42,43), except in TBI. Responses to the crystalloid challenge are used to group the patient into one of three categories of IVF challenge response: rapid response, transient response, no response. Transient and no responders to the first crystalloid bolus may require blood product transfusion and surgical care at the receiving trauma center.
Hemorrhagic Shock but no vital organ dysfunction: consider “permissive hypotension”
If the bleeding trauma patient with a lower SBP demonstrates adequate vital organ perfusion (appropriate mentation), then a “permissive hypotension” (44,45) strategy may be considered, wherein a SBP of 70-100 (3) is allowable until definitive surgical hemorrhage control is possible. Small fluid challenges (NS 250cc IV) (46) may be given to maintain SBP goals and vital organ perfusion. Use of permissive hypotension may be limited by time delays to definitive bleeding control at the receiving trauma center. Discuss the use of permissive hypotension with your receiving trauma consultant. Importantly, permissive hypotension should not be pursued in patients with TBI, spinal cord injury (47), severe cardiovascular disease, and pediatric patients, and this strategy has primarily been evaluated in patients with penetrating injury. Hypotension in children is an indicator of a decompensated shock state.
Fluid Resuscitation in Pediatric Hemorrhagic Shock
Sick pediatric trauma patients suffer many of the same negative consequences of the lethal triad, worsened by crystalloids (36). Pediatric MTP’s are utilized in many trauma centers with early use of balanced blood products (48). Currently, there is no consensus on the best balance of ratio of blood products in pediatrics (49). In children demonstrating hemodynamic compromise, ATLS 9th ed. recommends an IV crystalloid 20cc/kg challenge. If the challenge does not improve hemodynamics, ATLS recommends starting O negative PRBC 10mL/kg. However, similar to adults, PRBC’s and FFP are optimal choices in pediatric patients. Discuss IVF resuscitation strategies with your pediatric trauma consultant.
Shock Resuscitation Goals
Markers for resuscitation success are limited in the time-limited acute setting. Restoration of vital organ perfusion and SBP >70 are the immediate resuscitation goals. Improved brain perfusion is suggested by improving mentation. Adequate renal perfusion is indicated by restoring output to normal amounts: adults 0.5mL/kg/hr, children >1-year-old 1mL/kg/hr, children <1 year old 2mL/kg/hr. (41). Other markers of resuscitation may include improvement of abnormal vital signs, shock index, and overall organ perfusion (improved skin color, skin warmth, peripheral pulses). Decreasing elevated serum lactate by 20% within the first two hours of care is associated with decreased mortality (50).
Reversing coagulopathy in bleeding trauma patients
Suspected coagulopathy of trauma may be reversed by blood product replacement and medications, such as TXA. TXA may decrease mortality of bleeding trauma patients if given within 3 hours of arrival, but the most benefit is realized within one hour (51,52). The dose is 1 gram IV over 10 minutes, followed by 1 gram IV over 8 hours. In children, TXA demonstrated decreased mortality with significant abdominal or extremity trauma and metabolic acidosis. (53) The pediatric dose is 15 mg/kg IV over 10 minutes (maximum 1 gram), then 2 mg/kg/hr x 8 hours. Another medication, rFVIIa, does not currently have enough data to render a recommendation for or against its use. (38).
Traumatic Brain Injury (TBI)
TBI may be suspected based on physical exam. TBI in children, especially <3 months old may occur, with minimal external trauma (54). Resuscitation goals are slightly different in those who are suspected to have significant TBI. Correct hypotension (permissive hypotension contraindicated) to maintain MAP of 80mmHg in the adult (2) and normal blood pressure for age in the child (55). Normal saline or hypertonic saline may be used, although there is no added benefit of hypertonic saline over NS (56,57) in patients without evidence of herniation. Prevent hypoxia and hypoglycemia (55). Institute ICP mitigation measures. If possible, elevate the head of bed or place bed in reverse Trendelenburg position. The patient’s head should be facing forward to prevent any blockage of blood flow out of the brain from the jugular venous system. Hyperosmolar therapy with either mannitol or Hypertonic Saline (HTS) may be considered for concern of increased ICP. However, one systematic review was unable to support the routine use of mannitol in severe TBI (58). Mannitol is a diuretic, so be very cautious of volume depletion. Consider placing a urinary catheter. Mannitol dosing for the adult is 1gram/kg IV and 0.5-1gram/kg for children (but not often used in children). Likewise, HTS may not be helpful in TBI, but also does not demonstrate harm. (59). The adult dose of HTS 3% is 250mL bolus, followed by infusion at 50mL/hr, and the pediatric dose of HTS 3% is 3mL/kg IV bolus. The decision to give hyperosmolar agents may be made in coordination with the trauma consultant. Induced hypothermia and hyperventilation are not routine acute interventions (60).
Radiology
In addition to E-FAST above, chest x-ray should be obtained to assess for traumatic injuries that need stabilization before transport (PTX). If time allows, AP pelvis x-ray can assess for bony injury and possible associated pelvic hemorrhage. Radiopaque entry wound markers may be helpful. Limit other studies unless necessary, and ensure they do not delay transfer. Some CT scanners may acquire images slowly, and obtaining radiologist reports may be delayed.
Laboratory (some of these will not be available in the non-trauma center)
Point of care glucose is helpful in altered mentation, especially in injured children. Other labs are not essential for transfer but may be ordered as time allows. Hemoglobin may be obtained for baseline, but is not a reliable marker for blood loss in the acute setting and is not a trigger for transfusion, unless <7g/dL (61). Platelets, PT/INR, PTT will be helpful to monitor for coagulopathy. Elevated serum lactate (62) and elevated base deficit (63) from VBG (64) or ABG are associated with increased mortality in trauma. Of note, lactate levels are also elevated due to ethanol ingestion (65). Fibrinogen levels may decrease in coagulopathy. Viscoelastic studies (Thromboelastogram (TEG) or thromboelastometry (TEM)) help guide specific blood product replacement but are often not available.
Medications
Analgesia should be provided as safely possible. Provide tetanus immunization and antibiotics as needed. Although definitive imaging and coagulation study results are often not available, consider reversal of hypocoagulable states due to anticoagulant medications (or underlying hypocoagulable conditions (hemophilia, Von Willebrand disease)) in coordination with the trauma consultant.
Be prepared to provide trauma care beyond stabilization
Despite efforts to transfer the patient as soon as safely possible, very often you will have time to continue to your secondary trauma survey to identify non-life threatening injuries. Monitor end-organ perfusion and ongoing fluid resuscitation needs. Maintain awareness of how much blood product you have remaining. Monitor for development of coagulopathy (bleeding from tubes and IV’s, cutaneous bleeding). Prevent hypothermia. Order relevant radiology studies as time permits. Take action on important lab results, as needed. You will have to decide if you “have time” for additional labs and procedures and suture repair, fracture reductions. In children and adults, if non-accidental trauma or neglect is suspected, consider consulting the appropriate social services.
The patient’s personal effects (clothes, pocket contents) may be “evidence” for legal considerations later. Follow your hospital’s “chain-of-custody” protocol as possible, or secure patient belongs with minimal tampering and transfer with the patient (if not secured by law enforcement). If applicable and feasible, avoid washing off residue from patient’s hands, as it may be evidence, too.
When the transport team arrives, make an effort to provide as much history and care information as possible. Send digital images of radiographic studies as well as copies of test and radiograph results with the patient. Send blood products with patient, as needed. Secure all tubes and wires. Keep patient warm. Whether the patient is alert or intubated, inform them what is happening and give them encouragement and hope. Ensure the patient is adequately sedated if intubated (and paralyzed).
Communicate with the family or friends (as HIPAA allows)
Family and friends may arrive to the hospital not knowing anything about the patient’s situation. Engage them with a sense of empathy and calm. Ensure they understand the care priorities at your non-trauma center ED: stabilize and transfer to the trauma center. You are not going to “find” every small fracture or skin abrasion; you are providing life-stabilizing care and making the patient as comfortable as possible. Reassure them that the trauma center team will provide on-going care.
Give the family clear directions to the destination transfer hospital. Give them an approximate time for patient arrival. Set expectations by informing them know they may have to wait in the waiting room until the trauma center team has completed their initial examinations, there may be several physicians from different specialties that may asking the same history questions, and the trauma center will be repeating physical exams and may be ordering more labs and radiographic studies.
After-action review/team debriefing
Trauma resuscitations may evoke strong emotional responses in ED staff, especially in pediatric trauma, often realized after the patient has transferred out of the ED. Even in a busy ED, attempt to take a minute to immediately debrief. Encourage honesty with thoughts/feelings, share them, and realize they are likely normal responses (because, after all, you are a human being). Remind your team it is “ok” (i.e., not a sign of weakness) to feel saddened or shocked by the particulars of a trauma case. Refresh your mind to care for the next patient, who probably will have no clue you just experienced a very stressful situation.
A review of the clinical aspects of the resuscitation is helpful to improve individual performance, team work, and hospital policy/practice. Attempt to identify practices to be sustained and those that need improvement. Again, many trauma patients “show up” to the non-trauma designated ED without prior notice, not allowing time to prepare for patient arrival. It is helpful for ED’s to be “pre-prepared” with essential trauma equipment that is on “stand-by”, or ready to be used in such unexpected trauma patient encounters. ED protocols should allow periodic equipment function checks (changing batteries, bulbs, cleaning) and checking for expiration dates. Additionally, periodic trauma simulations and inservices involving all ED personnel are valuable to increase knowledge, improve team work, and develop “muscle memory” of the steps of a resuscitation.
Case resolution
After your team dawns protective gear, you calmly direct one nurse to the patient’s right side to remove the patient’s clothes and obtain vital signs and the other nurse to the patient’s left to start a large bore IV. Given his altered mentation, you anticipate the need for intubation. You ask your RT to gather intubation equipment and begin pre-oxygenating the patient. A cervical collar is placed. HR is 120 and BP 60/30. Blood replacement will likely be needed. You ask the charge nurse to bring two units of PRBC’s and TXA 1 gram. FFP will take 20 minutes to thaw. Your primary survey reveals a laceration to the left upper arm with pulsatile bleeding not controlled with direct pressure. You place a tourniquet, which stops the arterial bleeding. Before RSI, you improve the shock index with rapid PRBC transfusion through the IV fluid warmer and perform a brief neurologic exam (disability exam). You intubate on first attempt and place an OG tube. You start a ketamine infusion for sedation and analgesia. The remainder of primary survey, including back and junctional areas, are normal. E-FAST is negative. You prevent hypothermia by placing warm blankets on the patient. Bedside chest x-ray reveals ET tube in appropriate position and no other injuries and normal AP pelvis x-ray. You ask the secretary to quickly make digital x-ray copies for the transfer packet. Vital signs are improving as you start the second unit of PRBC’s. The trauma center surgeon returns your call and accepts the patient, advising PRBC transfusion to keep SBP between 70 to 80 mmHg. You order a tetanus vaccination, cephalexin 1gram IV, and start your secondary exam as the transport team arrives. After providing a good patient care report, you ensure tubes are secured, and send all patient belongings, patient chart, radiographs, and two extra units of blood with the patient. After the patient leaves, you and your team conduct a brief after action review and answer questions. The team is grateful for your resiliency and calm in an unexpected sick trauma patient encounter.
References / Further Reading:
- Madigan MC, Kemp CD, Johnson JC, et al. Madigan Secondary abdominal compartment syndrome after severe extremity injury: are early, aggressive fluid resuscitation strategies to blame? J Trauma. 2008 Feb; 64(2):280-5.
- Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016; 20: 100.Published online 2016 Apr 12. doi: 10.1186/s13054-016-1265-x. PMCID: PMC4828865
- Mizobata Y. Damage control resuscitation: a practical approach for severely hemorrhagic patients and its effects on trauma surgery. Journal of Intensive Care20175:4
- Brown JB, Cohen MJ, Minei JP, et al. Goal-directed resuscitation in the prehospital setting: a propensity-adjusted analysis. J Trauma Acute Care Surg. 2013 May; 74(5):1207-12; discussion 1212-4.
- Chang R, Holcomb MB. Optimal Fluid Therapy for Traumatic Hemorrhagic Shock Critical Care Clinics. 2017 Jan; 33(1):15-36.
- Giannoudi M, Harwood P. Damage control resuscitation: lessons learned. Eur J Trauma Emerg Surg. 2016; 42: 273–282.
- Interfacility Transfer of Injured Patients: Guidelines for Rural Communities. https://www.facs.org/~/media/files/quality%20programs/trauma/publications/ruralguidelines.ashx. Accessed August 25, 2017.
- Marcin JP, Pollack MM: Triage scoring systems, severity of illness measures, and mortality prediction models in pediatric trauma. Crit Care Med 30: S457, 2002. [PMID: 12528788]
- http://cotccc.com/wp-content/uploads/TCCC-Guidelines-for-Medical-Personnel-170131.pdf accessed August 19, 2017.
- Beekley AC, Sebesta JA, Blackbourne LH, et al. 31st Combat Support Hospital Research Group. Prehospital tourniquet use in Operation Iraqi Freedom: effect on hemorrhage control and outcomes. J Trauma. 2008;17(2 Suppl):S28–37.
- Sakles, JC, Mosier JM, Patanwala AE, et al. First pass success without hypoxemia is increased with the use of apneic oxygenation during rapid sequence intubation in the emergency department. Acad Emerg Med. 2016 Jun;23(6):703-10.
- Weingart SD, Trueger NS, Wong N, et al. Delayed sequence intubation: a prospective observational study. Ann Emerg Med. 2015;65: 349-355.
- Heffner A, et al. Incidence and factors associated with cardiac arrest complicating emergency airway management. Resuscitation 84 (2013) 1500-1504.
- Heffner A, et al. Predictors of the complication of postintubation hypotension during emergency airway management. Journal of Critical Care (2012) 27, 587–593.
- http://www.acepnow.com/article/timing-resuscitation-sequence-intubation-for-critically-ill-patients/. Accessed September 4, 2017.
- Shiima Y. Predicting post-endotracheal intubation cardiac arrest in pediatrics: Crit Care Med. 2016 Sep;44(9):1675-82. doi: 10.1097/CCM.0000000000001741.
- Upchurch CP, Grijalva CG, Russ S, et al. Comparison of Etomidate and Ketamine for Induction During Rapid Sequence Intubation of Adult Trauma Patients. Ann Emerg Med 2017; 69 (1): 24.e2. doi: 10.1016/j.annemergmed.2016.08.009.
- Cohen, L, Athaide V, Wickham ME, et al., The effect of ketamine on intracranial and cerebral perfusion pressure and health outcomes: a systematic review. Ann Emerg Med, 2015. 65(1): 43-51 e2.
- Jabre P, Combes X, Lapostolle F. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet 2009; 374 (9686): 293-300.
- http://pemplaybook.org/podcast/adventures-in-rsi/. Accessed September 10, 2017.
- Wadia, S., Bhola R, Loranz D., et al., Ketamine and intraocular pressure in children. Ann Emerg Med, 2014. 64(4): 385-88.
- http://www.wikem.org/wiki/Needle_cricothyrotomy. Accessed Aug 19, 2017
- https://emcrit.org/wp-content/uploads/vent-handout.pdf accessed August 17, 2017.
- Knotts D, Arthur AO, Holder P, et al. Pneumothorax volume expansion in helicopter emergency medical services transport. Air Med J. 2013 May-Jun;32(3):138-43. doi: 10.1016/j.amj.2012.10.014.
- Caro, DA, Andescavage S, Akhlaghi M., et al. Pupillary Response to Light Is Preserved in the Majority of Patients Undergoing Rapid Sequence Intubation. Annals of Emergency Medicine. 2011 Mar; 57(3):234-37.
- Maegele M, Lefering R, Yucel N, et al. Polytrauma of the German Trauma Society (DGU). Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007 Mar; 38(3):298-304.
- Guly HR, Bouamra O, Spiers M, et al. Trauma Audit and Research Network. Vital signs and estimated blood loss in patients with major trauma: testing the validity of the ATLS classification of hypovolaemic shock. Resuscitation. 2011 May;82(5):556-9. doi: 10.1016/j.resuscitation.2011.01.013. Epub 2011 Feb 23.
- Mutschler M, Paffrath T, Wölfl C, et al. The ATLS(®) classification of hypovolaemic shock: a well-established teaching tool on the edge? Injury. 2014 Oct; 45 Suppl 3():S35-8.
- Caputo N, Fraser R, Paliga A, et al. Triage vital signs do not correlate with serum lactate or base deficit, and are less predictive of operative intervention in penetrating trauma patients: a prospective cohort study. Emerg Med J.2013 Jul;30(7):546-50. doi: 10.1136/emermed-2012-201343. Epub 2012 Jul 16.
- http://rebelem.com/ten-trauma-resuscitation-commandments/. Accessed September 12, 2017.
- Mutschler M, Nienaber U, Münzberg M. The Shock Index revisited – a fast guide to transfusion requirement? A retrospective analysis on 21,853 patients derived from the TraumaRegister DGU®. Crit Care. 2013; 17(4): R172. doi: 10.1186/cc12851. PMCID: PMC4057268
- ACS TQIP. Massive Transfusion in Trauma Guidelines. American College of Surgeons.Available at: https://www.facs.org/%7E/media/files/quality%20programs/trauma/tqip/massive%20transfusion%20in%20trauma%20guildelines.ashx. Accessed September 1, 2017.
- Savage SA, Zarzaur BL, Croce, MA, et al. Redefining massive transfusion when every second counts. Journal of Trauma and Acute Care Surgery. 2013 Feb; 74(2): 396–402. doi: 10.1097/TA.0b013e31827a3639
- Caputo ND, Kanter M, Fraser R, et al. Comparing biomarkers of traumatic shock: the utility of anion gap, base excess, and serum lactate in the ED. American Journal of Emergency Medicine, 2015 Sep; 33(9): 1134-39.
- Stone, ME Jr, Katlata S, Liveris A, et al. End-tidal CO2 on admission is associated with hemorrhagic shock and predicts the need for massive transfusion as defined by the critical administration threshold: a pilot study. 2017 Jan;48(1):51-57.
- Leeper C.M., Peitzman A., Gaines B.A. (2017) Principles of Damage Control for Pediatric Trauma. In: Pape HC, Peitzman A, Rotondo M, Giannoudis P. (eds) Damage Control Management in the Polytrauma Patient. Springer, Cham pp 233-247
- Diab Y.A., Wong E.C., and Luban N.L.: Massive transfusion in children and neonates. Br J Haematol 2013; 161: 15-26
- Cannon JW, Khan MA, Raja AS. Damage Control Resuscitation in Patients with Severe Traumatic Hemorrhage. J Trauma. 2017 Mar; 82(3):605-617.
- Holcomb JB, Tilley BC, Baraniuk S, Fox EE, et al; PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015 Feb 3;313
- Cannon JW, Johnson MA, Caskey RC, et al. High ratio plasma resuscitation does not improve survival in pediatric trauma patients. J Trauma Acute Care Surg.2017 Aug;83(2):211-217. doi: 10.1097/TA.0000000000001549.
- 41. ATLS Student Course Manual, 9thedition
- Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012 Jul; 256(1):18-24.
- Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012 Oct 17; 308(15):1566-72.
- Morrison CA, Carrick MM, Norman MA, et al. Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: preliminary results of a randomized controlled trial. J Trauma. 2011 Mar; 70(3):652-63.
- Brown JB, Cohen MJ, Minei JP, et al. Goal-directed resuscitation in the prehospital setting: a propensity-adjusted analysis. J Trauma Acute Care Surg 2013;74(5):1207–12.
- Schreiber MA, Meier EN, Tisherman SA. Et al. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: Results of a prospective randomized pilot trial. Journal of Trauma and Acute Care Surgery. 2015 Apr. 78(4): 687–97
- Berry C, Ley EJ, Bukur M, et al. Redefining hypotension in traumatic brain injury. Injury. 2012 Nov; 43(11):1833-7.
- Joint Trauma System Guidelines: Damage Control Resuscitation. http://www.usaisr.amedd.army.mil/cpgs/DamageControlResuscitation_03Feb2017.pdf. Accessed Aug 23, 2017.
- Tran A, Campbell BT. The art and science of pediatric damage control. Seminars in Pediatric Surgery. 2017 Feb. 26 (1): 21-26.
- Régnier MA, Raux M, Le Manach Y, et al. Prognostic significance of blood lactate and lactate clearance in trauma patients. Anesthesiology.2012 Dec;117(6):1276-88. doi: 10.1097/ALN.0b013e318273349d.
- CRASH-2 trial collaborators, Shakur H, Roberts I, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010 Jul 3;376(9734):23-32. doi: 10.1016/S0140-6736(10)60835-5. Epub 2010 Jun 14.
- Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg.2012 Feb;147(2):113-9. doi: 10.1001/archsurg.2011.287. Epub 2011 Oct 17.
- Eckert, MJ, Wertin TM, Tyner SD, et al. tranexamic acid administration to pediatric trauma patients in a combat setting: the pediatric trauma and tranexamic acid study (PED-TRAX). J Trauma Acute Care Surg. 2014 Dec;77(6):852-8.
- http://pedemmorsels.com/mild-closed-head-injury-the-3month-old-caveat/. Accessed Oct 2, 2017.
- https://emergencymedicinecases.com/pediatric-trauma-2/ accessed August 20, 2017.
- Bulger EM, May S, Kerby JD, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011 Mar; 253(3):431-41.
- Bulger EM, May S, Brasel KJ, et al. Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA. 2010 Oct 6; 304(13):1455-64.
- Gottlieb M, Bailitz J. Does Mannitol Reduce Mortality From Traumatic Brain Injury? Journal of EM. 2016 Jan; 67(1):83-85
- Berger-Pelleiter E, Emond M, Lauzier, et al. Hypertonic saline in severe traumatic brain injury: a systematic review and meta-analysis of randomized controlled trials. CJEM. 2016 Mar;18(2):112-20. PMID: 26988719.
- Stevens RD, Shoykhet M, Cadena R. Emergency neurological life support: intracranial hypertension and herniation. Neurocrit Care. 2015;23(Suppl 2):76–82. doi: 10.1007/s12028-015-0168-z.
- EAST Guidelines. Napolitano LM, Kurek S, Luchette FA, et al. Red Blood Cell Transfusion in Adult Trauma and Critical Care. J Trauma. 2009; 67(6): 1439-42.
- Baxter J1, Cranfield KR, Clark GE, et al. Do lactate levels in the Emergency Department predict outcome in adult trauma patients? A Systematic Review. J Trauma Acute Care Surg. 2016 Jun 8; (5):471-82.
- Davis JW, Parks SN, Kaups KL, et al. Admission base deficit predicts transfusion requirements and risk of complications. J Trauma. 1996 Nov; 41(5):769-74.
- Arnold TD, Miller M, van Wessem KP, et al. Base deficit from the first peripheral venous sample: a surrogate for arterial base deficit in the trauma bay. J Trauma. 2011;71(4):793–797. doi: 10.1097/TA.0b013e31822ad694.
- Herbert HK, Dechert TA, Wolfe L, et al. Lactate in trauma: a poor predictor of mortality in the setting of alcohol ingestion. Am Surg. 2011;77(12):1576–1579.








