emDOCs Revamp: Appendicitis
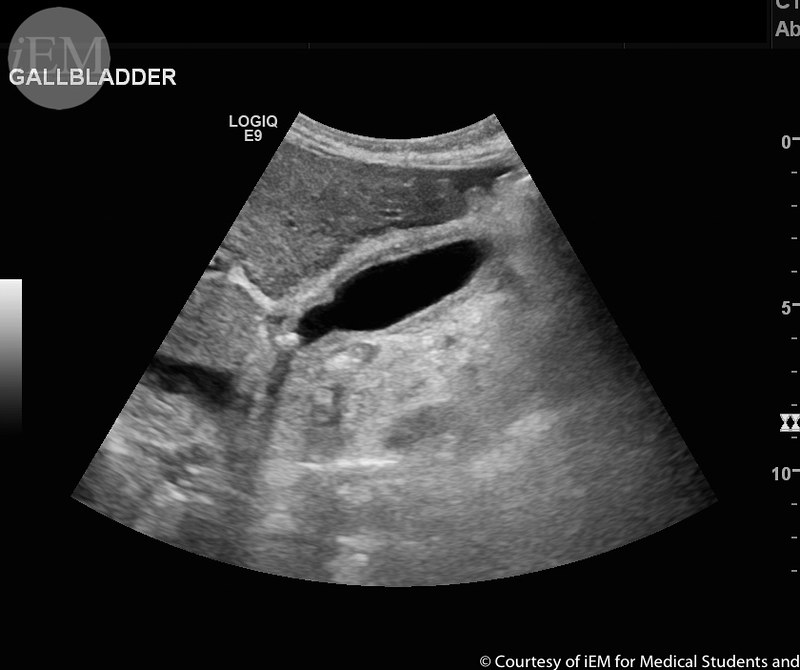
An 18-year-old female presents to the ED for abdominal pain. The pain began the day prior in the “middle of her stomach,” but is now “lower to the right” and associated with rigors and anorexia. She denies new sexual partners, dysuria, or vaginal discharge, and her last menses was 1 week prior. Triage Vital Signs: BP 109/69, HR 115, T 102.4°F Oral, RR 21, SpO2 99% on room air. She appears uncomfortable but with no acute distress. Her abdomen is soft. She has tenderness to palpation at McBurney’s point, tenderness in the right lower quadrant when palpating the left; evidence of involuntary guarding. What’s the next step in your evaluation and treatment?
emDOCs Revamp: Appendicitis Read More »