Clostridium difficile in the Emergency Department: Evaluation and Management
- Jul 29th, 2019
- Brandon Carius
- categories:
Authors: Brandon Carius, MPAS, PA-C (EMPA, San Antonio, Texas) and Brit Long, MD (EM Attending Physician, San Antonio, Texas) // Edited by: Alex Koyfman, MD (@EMHighAK)
Case #1
A 72-year-old woman presents with abdominal pain and increasing diarrhea over the past 48 hours. She describes 10 total episodes in the last 48 hours, her last approximately 1 hour before coming the emergency department, and tells you that they are mostly watery but some have seemed to have some blood. She denies any prior history of diarrhea similar to her current episodes. She recalls being discharged from the hospital after a 2-night stay for pneumonia approximately 2 weeks ago and finished the oral antibiotics she was started on (levofloxacin). Her heart rate is 110 beats per minute, blood pressure 108/68 (which she states is normal for her), has an SpO2of 99% on room air, and is afebrile with an oral temperature of 98.9°F. Her abdomen has generalized tenderness to palpation without findings of peritoneal signs. What is on your differential for this patient? What are your next steps?
Background
Clostridium difficile is an anaerobic, Gram-positive, spore-forming bacterium producing enterotoxins commonly associated with complaints of diffuse watery diarrhea classically blamed on antibiotic use and hospitalization. While C. difficile infections (CDI) are concerning, not all strains of are toxin producing, and the majority of CDI are nontoxigenic in nature that do not cause significant symptoms or require antibiotic treatment.1-3 Patients may also present less frequently with abdominal pain, fevers, or even simple nausea and vomiting, which can cloud the clinical picture for CDI versus a less worrisome viral illness. Although antibiotic use is a risk factor for CDI, lack of recent use should not be reassuring.
Rates of CDI have declined in Europe in previous years after hitting a peak in 2010, but the U.S. has continued to average approximately 500,000 cases annually.4,5 As of 2014, CDI has replaced MRSA as the most common agent causing hospital acquired infection in the U.S.6 Increasing numbers of high-quality studies and improved testing and surveillance have impacted the diagnosis and treatment of CDI. In response, several new practice guidelines have recently addressed CDI, including the Infectious Diseases Society of America (IDSA).7 Of note, there are no current policy statements on C. difficile diagnosis and treatment in the emergency department setting from organizations such as the American College of Emergency Physicians (ACEP) and American Academy of Emergency Medicine (AAEM) at the time of this writing.
Pearls: CDI is now the most common hospital-acquired infection in the U.S. Not all C. difficile strains are toxigenic. Several important updates have been published in research, diagnosis, and management, including by major societies, in recent years, but there are no significant policies on CDI from major emergency medicine organizations.
Risk Factors
Risk for CDI centers on two factors: risk of exposure and increased susceptibility to C. difficile. While the former centers primarily on routes of exposure and contact with contaminated persons or hospital areas, the latter includes medical factors such as recent antibiotic use, immunocompromised states, and even an association with proton pump inhibitors (PPI).
CDI has been primarily associated with the use of antibiotics, and this remains the most important risk factor for CDI development.7,8 Antibiotics disrupt the normal microbiota of the gut, which then allows C. difficile seeding in newly-exposed persons, or for previously-suppressed C. difficile spores to germinate and flourish unopposed in the GI tract. While all antibiotics carry some inherent risk of inducing CDI, several have been found to be of particular concern: beta-lactam and beta-lactamase inhibitors,9 third- and fourth-generation cephalosporins,8-10 fluoroquinolones,8,9,11,12 carbapenems,9,10 and clindamycin.8,9,13
Although not consistent across all studies, fluoroquinolones may have the greatest association with CDI, including one study demonstrating fluoroquinolone use in over half of the antibiotic prescriptions found in newly-diagnosed CDI.14 This may be due to newer ribotype 027 strains that demonstrate increased resistance to fluoroquinolones, which may play a role not only in increased virulence, but also increased fluoroquinolone-associated CDI. Although antibiotic exposure for longer than 10 days is associated with CDI, this duration is not necessary for CDI to occur. Even single doses of prophylactic antibiotics for surgical cases can increase CDI risk.15,16 Similarly, while antibiotic-associated CDI often involves recent medication use, literature has expanded “recent” to include up to 3 months after antibiotic initiation, regardless of its duration,7,14 making a patient’s historical recall all the more important in raising suspicion of the condition. Antibiotics with less association with CDI include macrolides, sulfonamides, and tetracyclines, although some level of risk remains.17
Patients with inflammatory bowel disease (IBD) conditions are high risk for developing CDI, especially those with ulcerative colitis (UC), who have greater than 3% risk of CDI within 5 years of UC diagnosis.18 This may be in part due to higher rates of asymptomatic CDI, compared to those without IBD.19,20 The use of immunosuppressive medications to control symptoms in IBD can also increase CDI risk.19,20
As documented CDI rates have increased in the past two decades, of particular concern is increased risk in the elderly.3,7,21,22 Rates of CDI in patients >65 years old have recently increased, with some studies showing an increase of 200%.5,21,23 One study found elderly patients had rates more than 5 times higher than any other age demographic.21 Although age-related immune system changes contribute, other age-related factors likely play a part, including increased rates of comorbidities, polypharmacy, increased hospital stay rates and length, and increased antibiotic usage.8
Hospitalization history is important in considering possible C. difficile exposure, but this should not be centered solely on medicine ward or similar stays. Authors have expanded the traditional hospital stay risk to include stays at nursing facilities, rehabilitation centers, and even assisted living centers.6,24 Similar to the expanded timeline of antibiotic use mentioned earlier, in some studies this has included a single overnight stay within the past three months of CDI diagnosis.14
Recent attempts have been made to categorize many of these risk factors into a single risk prediction model.25,26 Patient age, recent hospital stays, chronic conditions (such as inflammatory bowel disease, cancer, and liver disease), and antibiotic use are used to calculate CDI risk. Many of these variables were not defined beyond their general labels (i.e. ‘immunosuppression’ is not specific to certain medications or comorbidities), or to their time of existence prior to patient presentation (such as recently diagnosed IBD vs. a 20-year-old diagnosis). One model of calculated values based on patient variables and risk stratification is listed here (note: this calculator is not on MDCalc; you’re on your own for using a pen and paper!). These models have not been undergone significant validation, however, and there are no official recommendations for models or risk stratification predictions from the IDSA or other associations.
There is increasing interest regarding the relationship of proton pump inhibitor (PPI) use and CDI. Initial literature illustrated increased rates of community-acquired C. difficile diagnosis in patients on PPI therapy.27 Others demonstrated increased risk of CDI in the hospital setting with any gastric suppression agent, but most significantly with PPI.28-30 However, findings were likely confounded by other factors including: increased CDI testing rates during the periods of study,28-30 lack of control for patients with preexisting gastrointestinal disease and diarrhea,28 longer hospital stays in PPI patients,29 pre-study PPI use,30 and the general observational nature of the studies.28-30 Therefore, without a prospective RCT, gastric acid suppression agents including PPIs may be associated with CDI, but a causal relationship has never been proven.
Literature has found that not all patients fit the traditional antibiotic-and-hospitalization-centric risk models. One recent study found that 40% of CDI patients lacked a history of antibiotics, and an additional 18% had no recent healthcare exposures.31 Other risk factors that have been less examined but still hold increased risk for CDI include HIV status, recent GI surgery, tube feedings, malnutrition, obesity, female sex, and low serum albumin.14,32,33 In pediatric populations, gastric suppressive agents (PPIs, histamine blocking agents) and feeding patterns have demonstrated some association with C. difficile rates, but further studies are needed.3
Pearls: Antibiotic use and healthcare interactions remain the primary risk factors for CDI, although they are not universal in all patients. Immunocompromised states, primarily IBD, increase risk significantly. Other risk factors include age, recent surgery, tube feeding history, and malnutrition. While PPIs have demonstrated correlation to the development of CDI, no causal relationship has been proven.
Pitfalls: While antibiotic use and healthcare interactions are of primary concern, definitions vary. Antibiotic use has been expanded as far out as 3 months for increased CDI risk; do not be reassured that “it’s been a while” since the patient finished their last dose. Healthcare interactions can include outpatient visits, hospital stays in various levels of care, and long-term assisted-living facilities. Some studies have demonstrated that CDI can occur in significant rates even without these two classic factors.
Presentation
The classic presentation of CDI is an unwell or ill-appearing patient complaining of diffuse, watery stools (with the clues of recent antibiotic use and/or hospitalization). The IDSA recommends clinicians consider C. difficile in a patient with a new onset of 3 or more unformed stools in a 24-hour period without explanation,7 and this standard is used throughout recent literature.3,13,23,34 The required number of stools has notably decreased from prior literature, where in the 1970s and 1980s authors were requiring 5-6 stools per 24 hour period to qualify patients for study inclusion.35-37 Stools may be largely watery, although a significant percentage of patients may present with bloody stools.38
Although diarrhea is central to the suspicion and diagnosis of CDI, it may not be an isolated symptom. Patients with CDI may also have vomiting, although some studies have excluded patients from C. difficile testing when they presented with diarrhea and vomiting out of concern for over-inclusion of viral gastroenteritis patients skewing results.14 Comorbidities such as a post-operative ileus may further complicate matters, as no bowel movements (unformed or otherwise) will be present.20 Delayed presentation of CDI with fulminant colitis, seen in approximately 1-3% of CDI cases, may likewise no longer have diarrheal symptoms.39,40 Suspicion should be increased in the setting of radiographic evidence of toxic megacolon or other severe distention in the absence of diarrhea.23
Pearls: Current literature supports a low threshold for CDI concerns in the presentation of a patient with diarrhea (>3 unformed stools in 24 hours). This necessitates clinical judgment and critical evaluation by providers, to include assessing for risk factors and relative likelihood of other causes.
Pitfalls: The indication for CDI testing is low and could encompass a broad range of patients. Be sure to use the overall clinical picture and risk factors to delineate proper testing populations. Likewise, do not be completely reassured in a patient without diarrhea or in which diarrhea has stopped, as this could be a complication of concomitant ileus or signal onset of megacolon.
Testing
Two concepts are primary for testing CDI in the emergency department setting: first, when to test, and second, how to test.
Who to test would seem obvious from IDSA guidelines of 3 or more unformed stools in a 24 hours period, but as discussed above, this may include patients with viral gastroenteritis and other non-CDI cases, which could significantly skew the consistency of your facility’s testing.7 Increased suspicion for the emergency clinician should come with simultaneous constitutional symptoms (fevers, chills, night sweats), as well as pertinent history of antibiotic use, comorbid conditions, and/or recent healthcare visits. The IDSA states that diarrheal symptoms not attributable to other conditions (such as IBD, tube feedings, chemotherapy, or laxative use) indicate the need for testing.7 As these comorbidities and treatments demonstrate increased risk for CDI as reviewed above, sensible clinical judgment must be used for when to order testing.
These principles of when to test become further contentious in pediatric patients, whose symptoms of fever, diarrhea, and abdominal pain may be more easily blamed on environmental exposures for rotavirus or norovirus at school or childcare, especially when diarrheal symptoms show 10 or more stools in a 24 hour period.41 In discussion with your local laboratory, however, most agree on recommendations that any non-liquid stool samples be rejected for testing, as they are less likely to indicate CDI.7,14
Following the first diagnosis and initial treatment of CDI, repeat testing ‘for cure’ or with a recent recurrence of symptoms is discouraged. Specifically, IDSA guidelines discourage repeat testing within the “same episode” of diarrheal symptoms (i.e. within 7 days).7 For other patients who present following treatment for CDI, judicious use of laboratory testing is important to avoid mistaking colonization for infection. Prior studies of routine surveillance demonstrate shedding of C. difficile pores and/or toxins for weeks or even months after clinical cure.42-45
Testing is largely localized to laboratories, but some principles are generally consistent throughout literature. CDI testing is centered on detection of the organism itself or the toxins (A and B) produced. Traditional use of the standard cell cytotoxicity neutralization assay (CCNA) has been largely abandoned because of a relatively slow, labor-intensive process.3,7 Glutamate dehydrogenase (GDH) immunoassays detect the metabolic enzyme that is found universally in all strains of C. difficile, but this method has low specificity given it cannot differentiate between toxigenic and nontoxigenic strains. With a rapid turn-around time and high specificity, the use of enzyme immunoassay (EIA) for toxins A and B is attractive.3 The downside to EIA for C. difficile toxins, however, is a relatively low sensitivity, with a high miss rate if used in isolation. In comparison to toxigenic culture (TC), EIA has shown poor sensitivity, (29-40%) but a much higher specificity of 99%.46 Compared to the slower, more labor intensive CCNA, improved sensitivities up to 92-99% have been shown as recently as 2016.46,47 Therefore, EIA is generally part of a two-step system, where a TC is grown with subsequent EIA testing on the isolate. CDI testing has evolved significantly over the past few years to include newer broad-spectrum PCR assays that can screen for bacterial and viral causes simultaneously in a single sample. In a recent head-to-head-to-head trial of 238 hospitalized patients suspected of having CDI, PCR detected C. difficile 47% more often than EIA, and 33% more often than bacterial cell culture.48 In some areas, these have doubled the detection of CDI in samples compared to culture and EIA methods.3,49
Overall, general consensus is lacking for which laboratory testing method is the best. IDSA guidelines have thus emphasized using a multistep algorithm with paired testing modalities where agreed upon by your laboratory office. Below is a summary of four main tests described.
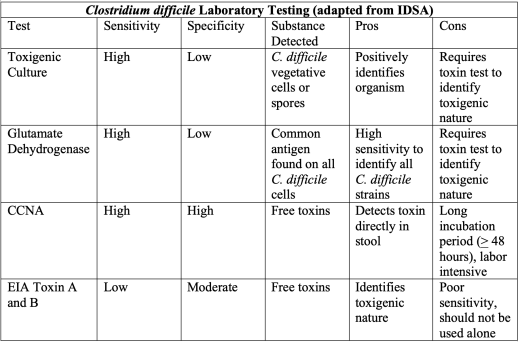
Pearls: Test patients with >3 unformed stools in a 24-hour period without a known cause, especially with risk factors of antibiotic use, hospitalization, or others as discussed previously. Clarify the ideal testing process with your local laboratory. Most utilize a multistep algorithm pairing TC and another test such as EIA. GDH requires similar EIA pairing to identify toxigenic strains, while CCNA has fallen out of favor due to length of time and labor requirements for testing. Newer PCR methods show promise.
Pitfalls: Do not use traditional CDI tests in isolation, as toxin-specific assays have low sensitivity and other tests have high sensitivity but do not delineate between toxigenic and nontoxigenic strains. Ensure you understand your department’s laboratory pathway for diagnosis and how best to employ it.
Categorization
Traditionally, CDI was classified as hospital-associated or community-associated, but more recently, a new group was added. There is a new three-way split of healthcare facility-onset (HO), community-associated (CA), and now community-onset, healthcare facility-associated (CO-HCFA) CDI. The definitions according to the Centers for Disease Control and Prevention (CDC) are below:50

Severity categorization for all patients are divided between mild and moderate-severe CDI. This has consistently been based upon two laboratory factors: white blood cell (WBC) count and kidney function (indicated by serum creatinine). Fulminant CDI is based upon patient hemodynamic status and presentation. Diagnostic cut-offs for these values are outlined below:

While these values are generally included in most literature, to include recent IDSA guidelines, they are not universal.7,51 Prior IDSA/SHEA guidelines in 2010 used a rise in serum creatinine of 1.5x baseline, and these are still used in surgical literature.20 The IDSA acknowledged that their change from a 1.5x rise from baseline creatinine to the stand alone 1.5 mg/dL creatinine level was based on practicality and an assumed lack of historical data. Other predictors of severe CDI may include a temperature above 38.5C (101.3F),20,51 albumin <2.5 g/dL,20,51 or hypotension.52
Additionally, fulminant CDI is categorized by WBC and creatinine levels above those of moderate-severe infection, with additional laboratory and physical examination findings, to include: shock or early vasopressor requirement, abdominal distention (concerning for ileus or megacolon), or elevated lactate (> 2 mmol/L).7,20 There is a consistent lack of definitive imaging recommendations based on presentation or for determinant diagnosis. Although there are no firm recommendations on imaging from the IDSA, the World Society of Emergency Surgery recommends that computed tomography (CT) “is suggested for patients with clinical manifestations of severe-complicated C. difficile colitis” without formerly defining “severe-complicated”.20 Conversely, some simply comment that CT imaging can help in determining the severity of the disease without any cited proof of benefit.53 Suspicion of fulminant colitis and megacolon is based largely on physical evaluation with concerns for peritoneal signs and unstable vital signs. Moderate-severe cases of CDI or those with systemic toxicity or complicated medical history should prompt consideration of imaging. Complications should include, but are not limited to: prior complicated CDI episodes, history of abdominal surgery or obstruction, and medical complications (such as diabetes, ulcerative colitis, or Crohn’s disease).
Patients with CDI may additionally be classified as having recurrent C. difficile infections, generally defined as having 3 or more episodes.52 It is important to note that diagnosis of recurrence can be blurred, however, as most occur with 7-14 days after completion of treatment, which leads to questioning of relapse versus a true recurrence.22,52,54 Continued exposure to C. difficile spores with likely poor hygiene is the primary contributing factor to true recurrence.22,52
Pearls: Categorization of CDI has grown from community- and healthcare facility-associated split to a third category of community-onset, healthcare facility-associated (CO-HCFA) CDI. Mild and moderate-severe CDI are categorized based on WBC and serum creatinine levels. The IDSA has recently changed recommendations from a 1.5x rise in baseline serum creatinine to an absolute serum creatinine level cut-off of 1.5 mg/dL, although some other guidelines still hold to the previous definition. Definitions of fulminant CDI vary based on other physical and laboratory findings, generally indicating an acute abdomen or organ damage.
Therapy
In cases of ongoing antibiotic use, discontinuation remains a mainstay of treatment. Although this strategy alone had previously proven effective in resolving CDI symptoms in 20-25% of patients within 72 hours,52 more virulent strains and resistance makes an isolated discontinuation strategy less logical. Treatment for diagnosed CDI used to be split based upon mild vs. moderate-severe categorization, with therapy mainstays of metronidazole and oral vancomycin, respectively. Metronidazole has never been approved by the FDA for use in CDI, however, and newer studies have largely overthrown metronidazole use with oral vancomycin and/or a newer option, fidaxomicin. For initial treatment of CDI, prior IDSA treatment strategies centered primarily on metronidazole for mild CDI and vancomycin for moderate-severe CDI were supplemented with two more recent RCTs since 2000 that found substantially improved rates of cure with oral vancomycin regimens.13,33 These trials are detailed below:

Although it can be used in initial CDI cases where vancomycin and fidaxomicin are unavailable, metronidazole is second-line, and patients who do not show improvement in symptoms during the first 1-2 days, or who worsen at any point during therapy, should be switched to oral vancomycin.52 Metronidazole is still recommended for initial therapy in CDI patients with fulminant presentation, given intravenously along with oral vancomycin for improved mortality benefit.7,55
Additionally, despite being replaced as a first-line medication in adult CDI, metronidazole is still a primary treatment option for the initial episode of mild pediatric CDI. Recent surveys of pediatric infectious disease physicians reveal 100% of respondents recommending metronidazole for initial CDI treatment in otherwise uncomplicated children, which is likewise supported by literature reviews and official recommendations from the American Academy of Pediatrics.3,7,56,57 For pediatric patients with comorbid conditions, or found to have severe CDI on initial presentation, oral vancomycin is recommended as primary therapy over metronidazole, although these patients can be considered for concomitant intravenous metronidazole with oral vancomycin.3,7,57
The macrolide fidaxomicin received FDA approval for use in C. difficile treatment in 2011 and is also recommended by the IDSA for initial CDI treatment.7 This recommendation is based on two large-scale RCT involving approximately 1100 patients that showed near-equal efficacy in clinical resolution of symptoms at 10 days between oral vancomycin and fidaxomicin.58,59 Fidaxomicin does not have approval for use in pediatric patients at this time, although some pediatric infectious disease physicians recommend its consideration in severe cases.56
Recommended treatment time for oral medication regimens is 10 days “to resolve symptoms in most patients”.7 Symptom improvement but failure for symptoms to fully resolve by day 10 warrants consideration to extend treatment to 14 days total.7 If using metronidazole in initial CDI treatment, providers should use caution in considering extending therapy due to neurotoxic concerns, although this warning is based largely on case reports from patients with liver disease.7,52,60-62 Although dosing regimens are adjusted for parental vancomycin, no such changes are recommended for oral dosing, as systemic absorption is low. Likewise, no renal dosing adjustments are recommended for fidaxomicin.
Previously, recommendations to treat recurrent CDI were to continue using the same regimen as with the first episode.22,52 There is less support for this strategy at this time, especially in light of decreased support for metronidazole use.7 Rather, any patient previously on metronidazole should be switched to oral vancomycin or fidaxomicin. If fidaxomicin is being used for the first time as treatment, a 10-day regimen can be used, even if the patient was previously on metronidazole or vancomycin. Vancomycin treatment recommendations vary if it is being used for recurrent CDI treatment. Although largely unproven, in theory pulse dosing is intended to keep C. difficile vegetative spores “in check while allowing restoration of the normal microbiota”.7,52 Options for oral vancomycin include tapered or pulsed dosing, with examples from IDSA below:7

Other medications for CDI treatment, both initial and recurrent, include nitazoxanide, fusidic acid, rifaximin, tigecycline, and oral bacitracin. Various trials, case reports, and reviews have examined efficacy of these alternatives without consistent evidence to support their use, and none have FDA approval for CDI treatment at this time.7
When prescribing vancomycin, clinicians should remember that the classic ‘Red Man Syndrome’ occurring from massive histamine release can occur with oral administration as well, which has been documented previously.63 Management includes discontinuation of the medication, administration of antihistamine medications, and consultation with infectious disease personnel.
Concerning presentations of fulminant CDI necessitate early surgical consultation in addition to antibiotics. Toxic megacolon can quickly progress to perforation, peritonitis, and septic shock, and surgical evaluation should not be delayed during initiation of treatment.20,22,52 Surgical options may include ileectomy and colectomy.
Pearls: Prior recommendations of metronidazole for first-line treatment in mild CDI are now replaced with oral vancomycin in adults, although metronidazole may still be considered as first-line in otherwise uncomplicated pediatric patients and as an adjunct IV therapy in fulminant CDI with oral vancomycin. Moderate-severe CDI necessitates oral vancomycin or fidaxomicin. All initial courses of antibiotics are 10-day regimens. Regimens may be extended to 14 days in cases which show vast improvement of symptoms without complete resolution. Recurrent CDI should not be treated with the same regimen as previously recommended; instead, options include a vancomycin tapered and pulsed dosing regimen or combinations of other antibiotics (in consultation with infectious disease). Fulminant CDI necessitates early surgical consultation.
Prevention
As previously discussed, the colonization and spread of C. difficile is primarily through contaminated environments and poor hygiene. This involves monitoring patients and providers alike for proper protection and hand hygiene to prevent further spread. Although used frequently in the ED, alcohol-based hand sanitizers are less effective than handwashing with soap and water, as C. difficile spores are resistant to alcohol solutions and have been shown to be equivalent to no hand hygiene at all.3,64 Hand washing is superior in large part due to the mechanical aspect of scrubbing and is recommended for all providers after interacting with suspected or diagnosed CDI patients.
Pearls: Alcohol-based hand sanitizers are not sufficient to kill spores. Wear your protective gear and wash your hands thoroughly with soap and water after suspected or confirmed CDI patient encounters.
Clostridium difficile Key Pearls and Pitfalls
- CDI has replaced MRSA as the most common nosocomial infection in the U.S.
- Antibiotic use and hospitalization continue to be primary risk factors of concern, although some patients may not have either present in their recent history. “Recent” is not uniformly defined in literature, in some out to 3 months prior to presentation of symptoms. Additionally, “hospitalization” has been expanded to include nursing homes, assisted living facilities, rehabilitation centers, and even outpatient clinic visits.
- Testing pitfalls remain for cultures with high sensitivity and low specificity and immunoassays with high specificity but low sensitivity. A two-step algorithmic approach can be used based in your local laboratory to ensure proper recognition of C. difficile and delineation of toxigenic vs. nontoxigenic strain. Newer PCR testing modalities are increasing recognition rates.
- Categorization is generally based on:
- Suspected acquisition source – healthcare facility associated (HO-CDI), community acquired (CA-CDI), or community-acquired, healthcare-facility onset (CA-HCFO-CDI).
- Severity – mild (WBC <15k, Cr <1.5mg/dL), moderate-severe (WBC >15k, Cr>5mg/dL), fulminant (signs of shock, elevated lactate, toxic megacolon).
- Treatment has evolved from using metronidazole in cases of mild CDI to now using oral vancomycin as a first-line medication in all CDI presentations, regardless of severity. A newer consideration is fidaxomicin. Oral metronidazole is still recommended for consideration as first-line treatment in pediatric patients, and as an intravenous therapy adjunct along with oral vancomycin in cases of fulminant CDI. Recurrent CDI warrants consideration of tapered-pulsed vancomycin regimens (although there is weak evidence for these treatment modalities).
- Alcohol-based hand sanitizers do not effectively kill or remove C. difficile spores. Mechanical hand scrubbing with soap and water is effective for removing organisms and should be used, in conjunction with other personal protective equipment, for all suspected and confirmed CDI encounters.
From Dr. Katelyn Hanson and Hanson’s Anatomy:

References/Further Reading:
- Gerding DN, Meyer T, Lee C, et al. Administration of spores of nontoxigenic clostridium difficile strain M3 for prevention of recurrent C difficile infection: a randomized control trial. JAMA. 2015;313(17):1719-1727.
- Kato H, Kato N, Watanabe K et al. Application of typing by pulsed-field gel electrophoresis to the study of Clostridium difficile in a neonatal intensive care unit. J Clin Microbiol. 1994;32(9):2067-70.
- Khalaf N, Crews J, DuPont HL, & Koo HL. Clostridium difficile: an emerging pathogen in children. Discov Med. 2012;14(75):105-113.
- Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(Suppl 2):S88-92.
- Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
- Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387-90.
- McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1–e48.
- Reveles KR, Lee GC, Boyd NK, & Frei CR. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control. 2014;42(10):1028-32.
- Brown KA, Khanafer N, Daneman N, Fisman DN.Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 2013;57(5):2326-2332.
- Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67(3):742-8.
- Pepin J, Shaeb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-60.
- Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26(3):273-80.
- Johnson S, Samore MH, Farrow KA, et al. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med. 1999;341(22):1645-51.
- Abrahamian FM, Talan DA, Krishnadasan A, et al. Clostridium difficile infection among US emergency department patients with diarrhea and no vomiting. Ann Emerg Med. 2017;70(1):19-27.e4
- Al-Obaydi W, Smith CD, Foguet P.Changing prophylactic antibiotic protocol for reducing Clostridium difficile-associated diarrhoeal infections. J Orthop Surg (Hong Kong). 2010;18(3):320-3.
- Privitera G, Carpelini P, Ortisi G, et al. Prospective study of Clostridium difficile intestinal colonization and disease following single-dose antibiotic prophylaxis in surgery.Antimicrob Agents Chemother. 1991;35(1):208-10.
- Oldfield EC IV, Oldfield EC III, Johnson DA. Clinical update for the diagnosis and treatment of Clostridium difficile infection. World J Gastrointest Pharmacol Ther. 2014;5(1):1-26.
- Negron ME, Rezaie A, Barkema HW, et al. Ulcerative colitis patients with Clostridium difficile are at increased risk of death, colectomy, and postoperative complications: a population-based inception cohort study.Am J Gastroenterol. 2016;111(5):691-704.
- Clayton EM, Rea MC, Shanahan F, et al. The vexed relationship between Clostridium difficile and inflammatory bowel disease: an assessment of carriage in an outpatient setting among patients in remission.Am J Gastroenterol. 2009;104(5):1162-9.
- Sartelli M, Di Bella S, McFarland LV, et al. 2019 update of the WSES guidelines for management of Clostridioides (Clostridium) difficile infection in surgical patients. World J Emerg Surg. 2019;14:8.
- McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerging Infectious Disease. 2006 March;12(3):409-15.
- Pepin J, Routheir S, Gagnon S, Brazeau I. Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada. Clin Infect Dis. 2006;42(6):758-64.
- Mizusawa M, Doron S, Gorbach S.Clostridium difficile diarrhea in the elderly: current issues and management options. Drugs Aging. 2015;32(8):639-47.
- Jump RL, Donskey CJ. Clostridium difficile in the Long-Term Care Facility: Prevention and Management. Curr Geriatr Rep. 2015;4(1):60–69.
- Kuntz JL, Johnson ES, Raebel MA, et al. Predicting the risk of Clostridium difficile infection following an outpatient visit: development and external validation of a pragmatic, prognostic risk score.Clin Microbiol Infect. 2015;21(3):256-62.
- Kuntz JL, Smith DH, Petrik AF, et al. Predicting the Risk of Clostridium difficile Infection upon Admission: A Score to Identify Patients for Antimicrobial Stewardship Efforts. Perm J. 2016;20(1):20–25.
- Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents, and the risk of community-acquired Clostridium difficile-associated disease. 2005;294(23):2989-95.
- Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003;54(3):243-5.
- Dial S, Alrasadi K, Manoukian C, et al.Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. 2004;171(1):33-8.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784-90.
- Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. 2013;173(14):1359-1367.
- Bliss DZ, Johnson S, Savik K, et al. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129(12):1012-9.
- Gourdazi M, Seyedjavadi SS, Goudarzi H, et al. Clostridium difficile infection: epidemiology, pathogenesis, risk factors, and therapeutic options. Scientifica (Cairo). 2014;2014:916826.
- Zar FA, Bakkanagari SR, Moorthi KM et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307.
- Fekety R, Silva J, Kauffman C, et al. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. Am J Med. 1989;86(1):15-9.
- Teasley DG, Gerdin DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. 1983;2(8358):1043-6.
- Tedesco FJ, Barton RW, Alpers DH.Clindamycin-associated colitis. A prospective study. Ann Intern Med. 1974;81(4):429-33.
- Morinville V, McDonald J. Clostridium difficile-associated diarrhea in 200 Canadian children. Can J Gastroenterol. 2005;19(8):497-501.
- Flegel W, Muller F, Daubener W, et al.Cytokine response by human monocytes to Clostridium difficile toxin a and toxin b. Infect Immun. 1991;59(10):3659-66.
- Welfare MR, Lalayiannis LC, Martin KE, et al. Comorbidities as predictors of mortality in Clostridium difficile infection and derivation of the ARC predictive score. J Hosp Infect. 2011;79(4):359-63.
- Gogate A, De A, Nanivandekar R, et al. Diagnostic role of stool culture & toxin detection in antibiotic associated diarrhea due to Clostridium difficile in children. Indian J Med Res. 2005;122(6):518-524.
- Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-55.
- McFarland LV, Elmer GW, Surawicz CM.Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769-75.
- Gerding DN, Johnson S, Peterson LR. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-77.
- Poutanen SM, Simor AE.Clostridium difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-8.
- Crobach MJ, Planche T, Eckert C, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2016;22(Suppl 4):S63-81.
- Planche T, Aghaizu A, Holliman R, et al. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review.Lancet Infect Dis. 2008;8(12):777-784.
- Chankhamhaengdecha S, Hadpanus P, Aroonnual A, et al. Evaluation of multiplex PCR with enhanced spore germination for detection of Clostridium difficile from stool samples of the hospitalized patients. Biomed Res Int. 2013;2013:875437.
- Luna RA, Boyanton BL, Mehta S, et al. Rapid stool-based diagnosis of Clostridium difficile infection by real-time PCR in a children’s hospital.J Clin Microbiol. 2011;49(3):851-7.
- Centers for Disease Control and Prevention. Clostridioides difficile Infection (CDI) Tracking. Available at: https://www.cdc.gov/hai/eip/cdiff-tracking.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fhai%2Feip%2Fclostridium-difficile.html. Accessed 12 June 2019.
- Bauer MP, Hensgens MP, Miller MA, et al. Renal failure and leukocytosis are predictors of a complicated disease course of Clostridium difficile infection if measured on day of diagnosis. Clin Infect Dis. 2012;55(Suppl 2):S149-153.
- Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S32-42.
- Napolitano LM, Edmiston CE Jr.Clostridium difficile disease: diagnosis, pathogenesis, and treatment update. 2017;162(2):325-48.
- Aas J, Gessert CE, Bakken JS.Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36(5):580-5.
- Rokas KE, Johnson JW, Beardsley JR, et al.The addition of intravenous metronidazole to oral vancomycin is associated with improved mortality in critically ill patients with Clostridium difficile infection. Clin Infect Dis. 2015;61(6):934-41.
- Sammons JS, Gerber JS, Tamma PD, et al.Diagnosis and management of Clostridium difficile infection by pediatric infectious diseases physicians. J Pediatric Infect Dis Soc. 2014;3(1):43-8.
- Schutze GE, Willoughby RE; Committee on Infectious Diseases; American Academy of Pediatrics. Clostridium difficile infection in infants and children. 2013;131(1):196-200.
- Cornely OA, Crook DW, Esposito R, et al; OPT-80-004 Clinical Study Group. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;55(Suppl 2):S93-103.
- Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection.N Engl J Med. 2011;364(5):422-31.
- Kapoor K, Chandra M, Nag D, et al.Evaluation of metronidazole toxicity: a prospective study. Int J Clin Pharmacol Res. 1999;19(3):83-8.
- Yamamoto T, Abe K, Anjiki H, et al. Metronidazole-induced neurotoxicity developed in liver cirrhosis. J Clin Med Res. 2012;4(4):295–8.
- Knorr JP, Javed I, Sahni N, et al. Metronidazole-induced encephalopathy in a patient with end-stage liver disease. Case Reports Hepatol. 2012;2012:209258.
- Arroyo-Mercado F, Khudyakov A, Chawla GS, et al.Red man syndrome with oral vancomycin: a case report. Am J Med Case Rep. 2019;7(1):16-17.
- Oughton MT, Loo VG, Dendukuri N, et al. Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for the removal of Clostridium difficile.Infect Control Hosp Epidemiol. 2009;30(10):939-44.
